- Review
- Open access
- Published:
Emerging insights into epigenetics and hematopoietic stem cell trafficking in age-related hematological malignancies
Stem Cell Research & Therapy volume 15, Article number: 401 (2024)
Abstract
Background
Hematopoiesis within the bone marrow (BM) is a complex and tightly regulated process predominantly influenced by immune factors. Aging, diabetes, and obesity are significant contributors to BM niche damage, which can alter hematopoiesis and lead to the development of clonal hematopoiesis of intermediate potential (CHIP). Genetic/epigenetic alterations during aging could influence BM niche reorganization for hematopoiesis or clonal hematopoiesis. CHIP is driven by mutations in genes such as Tet2, Dnmt3a, Asxl1, and Jak2, which are associated with age-related hematological malignancies.
Objective
This literature review aims to provide an updated exploration of the functional aspects of BM niche cells within the hematopoietic microenvironment in the context of age-related hematological malignancies. The review specifically focuses on how immunological stressors modulate different signaling pathways that impact hematopoiesis. Methods: An extensive review of recent studies was conducted, examining the roles of various BM niche cells in hematopoietic stem cell (HSC) trafficking and the development of age-related hematological malignancies. Emphasis was placed on understanding the influence of immunological stressors on these processes.
Results
Recent findings reveal a significant microheterogeneity and temporal stochasticity of niche cells across the BM during hematopoiesis. These studies demonstrate that niche cells, including mesenchymal stem cells, osteoblasts, and endothelial cells, exhibit dynamic interactions with HSCs, significantly influenced by the BM microenvironment as the age increases. Immunosurveillance plays a crucial role in maintaining hematopoietic homeostasis, with alterations in immune signaling pathways contributing to the onset of hematological malignancies. Novel insights into the interaction between niche cells and HSCs under stress/aging conditions highlight the importance of niche plasticity and adaptability.
Conclusion
The involvement of age-induced genetic/epigenetic alterations in BM niche cells and immunological stressors in hematopoiesis is crucial for understanding the development of age-related hematological malignancies. This comprehensive review provides new insights into the complex interplay between niche cells and HSCs, emphasizing the potential for novel therapeutic approaches that target niche cell functionality and resilience to improve hematopoietic outcomes in the context of aging and metabolic disorders.
Novelty statement
This review introduces novel concepts regarding the plasticity and adaptability of BM niche cells in response to immunological stressors and epigenetics. It proposes that targeted therapeutic strategies aimed at enhancing niche cell resilience could mitigate the adverse effects of aging, diabetes, and obesity on hematopoiesis and clonal hematopoiesis. Additionally, the review suggests that understanding the precise temporal and spatial dynamics of niche-HSC interactions and epigenetics influence may lead to innovative treatments for age-related hematological malignancies.
Graphical abstract
Introduction
During the developmental periods, mesenchymal stem cells (MSCs) and other stem cells exhibit microheterogeneity, specific series of temporal events [1]. The significant intricate potential of these cells in tissue repair, and cell replacement makes remarkable development of stem cell-based treatments, which require substantial preclinical and clinical studies. However, the unproven efficacy of these cells to ameliorate patient maladies successfully is questionable in terms of science and medical applications.
The bone marrow is a complex and vital component of the human body. It has a diverse and dynamic environment consisting of characteristic cells that work in unison to produce blood and immune cells. The bone marrow houses hematopoietic stem cells (HSCs) and multipotent progenitors (MPPs). Bone marrow niche is perpetually dynamic, where billions of cells are produced to replace transient cells. These are periodically cleared and create an endless supply of blood factors and cells necessary for oxygenation and homeostatic regulation. Bone marrow possesses a unique “demand-adapted” hematopoiesis which is triggered during injury or infection, where there is a surge in production and levels of high-demand cells. This sheds light on the inflammatory response pathway of bone marrow [2]. The bone marrow (BM) microenvironment is highly vascularized and composed of several niche components such as stromal and nonstromal cells. Niche components inside the BM could act directly on the hematopoietic stem cells (HSCs). For instance, the perivascular cells are confined to the BM and exemplified by the expression of Nestin + mesenchymal stem cells (MSCs). These cells can be categorized into Nestin-GFP bright and Nestin-GFPdim in Nestin-GFP transgenic mice. The Nestin-GFPdim cells are confined to the sinusoids whereas the Nestin-GFPbright cells are confined to the arterioles [3, 4]. Stromal cells are categorized into NG2 + cells CXCL-12 abundant reticular cells [5], and leptin receptor cells [6,7,8]. Stem cells confined to the BM could undergo self-renewal and generate a progeny of lineages such as myeloid and lymphoid cells. Mainly, HSCs maintain contact with the osteoblasts in the sinusoidal endothelium in the BM microenvironment. Several anchoring cells are evident in the BM to foster HSCs’ survival, retention, and proliferation. Specifically, endothelial cells (ECs) and MSCs are supporting cells that can promote the survival of HSCs and subsequently foster BM regeneration. Furthermore, sympathetic and parasympathetic innervations of the autonomic system onto the BM could affect the hematopoietic system.
Aging [9], diabetes [10, 11], obesity-induced proinflammatory states [12], and the formation of fatty BM [12] are a few potential reasons for clonal hematopoiesis of intermediate potential (CHIP)-associated pathological conditions [12]. In this review, we have discussed the updated mechanistic role of several niche cells in the BM in the promotion of HSCs proliferation and differentiation upon aging-induced genetic/epigenetic changes, and metabolic diseases in order to target age-related haematological malignancies. We discussed the role of several biological and pharmacological molecules in modulating the HSPCs trafficking in both hematopoiesis during aging-related hematological malignancies by modulating several signaling pathways.
Literature search
We conducted a comprehensive literature review by gathering data from various databases, including Pubmed, Medline, eMedicine, Scopus, Google Scholar, the National Library of Medicine (NLM), and ReleMed. We focused on published reports and articles spanning from 1950 to 2023, exploring studies related to the implications of niche cells within the bone marrow (BM) on hematopoiesis, the influence of neural signaling on hematopoiesis during hematological malignancies while addressing immunological stressors, and their potential clinical implications. Key search terms such as “aging,” “obesity,” “diabetes,” “hematopoiesis,” “BM niche cells,” “epigenetics/genetics”, “clonal hematopoiesis,” “clonal hematopoiesis of indeterminate potential (CHIP),” “immune stressors,” and “hematological malignancies” were utilized to ensure effective literature collection. Relevant articles focusing on the implications of BM niche cells on hematopoiesis during hematological malignancies, particularly in the context of overcoming immunological stressors, were identified for analysis.
BM microenvironment
The bone marrow microenvironment is composed of several different kinds of cell types including hematopoietic stem cell progenitors, osteoblasts, immune cells, osteoclasts, and perivascular cells [13, 14]. Primitive hematopoietic cells undergo trafficking to the specific vascular regions of the bone marrow where CXCL12 and E-selectin are abundant. Different kinds of MSCs can regulate the HSCs’ survival in the specialized bone niches. However, the significant diversity and the lineage relationships are yet to be explored vividly for MSCs in the BM. The majority of them are confined across the perivascular space arterioles, and sinusoids and generate several significant niche factors such as SCF and CXCL-12. Consequently, these factors are identified by the leptin receptor [15, 16], Nestin [17], or NG2 (Cspg4) [3], these were explored by studying the Lepr-cre and Nes-GFP reporter non-inducible mouse lines respectively [17, 18]. Bone marrow damage is evident in chemotherapy or radiotherapy after irradiation. Irradiation could damage sinusoids across the bone marrow [13, 19, 20] and the arteriolar blood vessels are preserved in the endosteum. However, it is yet to be examined the role of these factors such as SCF and CXCL-12 in producing either distinct or overlapping cell lineages during hematopoiesis across BM.
Hematopoiesis
HSCs are multipotent primitive cells that can differentiate into different kinds of blood cells such as myeloid-lineage and lymphoid-lineage cells [21]. These cells can be abundant in peripheral blood, and bone marrow. These lineages are significantly generated through the self-renewing multipotent HSCs. A vivid understating of the self-renewal and differentiation mechanisms could have significant clinical implications pertinent to disease type and severity. The minimal amount of this HSC population can foster hematopoiesis [22]. Murine models: Previous studies described the long-term dormancy and quiescence of the HSCs is mediated by several genetic and epigenetic factors as described in the studies related to p21cip1/waf1 by using p21-/- mice [23,24,25]. HSCs confined to the bone marrow are significantly involved in the generation of all kinds of blood cells. Albeit HSCs undergo division infrequently, it is suggested that the HSC pool turns over randomly depending on the internal cues and they often undergo division through entering/exiting the cell cycle.
Previous studies have delineated the flow cytometry-based label-retaining assays using BrdU and histone H2B-GFP to observe a specific population of dormant HSCs in murine models within linSca1 + cKit + CD150 + CD48CD34 population [26]. In addition, computational models described that the dormant HSCs undergo division every 145 days and 5 times in their lifetime [26]. Stimulation through G-CSF activity or bone marrow injury could induce self-renewal HSCs suggesting their efficacy to switch from dormancy to self-renewal at the time of hematopoietic stress [26]. As soon as they re-established the homeostasis, they can return to dormancy suggesting that HSCs are not stochastically undergoing the cell cycle but undergo a reversible switch from dormancy to self-renewal at the time of hematopoietic stress [26].
Several transplantation studies described that the HSCs can live longer when compared to the donor from which they specifically isolated [27]. Similar to stem cells, telomerase in the HSCs is significantly involved in the stability of chromosomes at the time of cell division [28]. Furthermore, the HSCs of aged donors are significantly different from young donors [29, 30]. For instance, HSCs of aged donors exhibit significantly a rapid cell cycle, altered cell surface phenotypic immune markers, and generate a higher number of myeloid cells, and these HSCs are associated with minimal efficacy in homing to bone marrow when compared to younger individuals [31,32,33,34,35,36]. During aged conditions, the HSCs exhibit comparatively a minimal self-renewal capacity when compared to their younger counterparts due to the DNA damage in HSCs [37, 38]. However, future studies are required to explore the age-related changes in the behavior of HSCs. Different subsets of HSCs exhibit distinct characteristics such as self-renewal capacities, repopulation kinetics, and differentiation capacities [39,40,41,42]. The clonal analysis described the HSC compartment in aged individuals and observed the three kinds of HSC subsets reported in younger mice [39, 43, 44]. Normally, the generation of myeloid and lymphoid cells is in a similar ratio to the HSCs self-renewal process in the blood of unmanipulated mice. Myeloid-biased HSCs could produce a minimal number of subsequent generations of lymphoid progeny whereas the lymphoid-biased HSCs could produce a minimal number of myeloid cells [39]. A minimal number of prethymic T-cell precursors as well as B-cell precursors are produced by the myeloid-biased HSCs. Significantly a slow response of lymphoid progeny has been observed to the IL-7 lymphokine at the time of minimal lymphopoiesis. Myeloid-biased HSCs from young murine models suggested a delay in the onset of repopulation after transplantation but could be conducive to profoundly longer peripheral hematopoiesis when compared to the other kinds of HSCs [39, 45].
MSCs
MSCs derived from the bone marrow are considered rare hematopoietic cells with self-renewal capacity to undergo differentiation into bone, cartilage, and fat. These cells could surround the arterioles and form wrapping loosely around the sinusoidal vessels. Furthermore, along with HSCs, the MSCs can form the CFC-Fs or mesenchymal spheres in in vitro conditions. During the transplantation of MSCs, they could be attributed to the ectopic formation of the hematopoietic niche consequently we can observe BM stromal cells and active haematopoiesis [46].
Murine models
Human bone marrow-derived CD146 + mesenchymal stem and progenitor cells (MSPCs) upon transplantation through subcutaneous route into the murine models have induced the generation of heterotopic hematopoietic niche associated with host-derived HSCs as well as donor-derived perivascular CD146 + MSPCs [47]. In addition, the transplantation of CD105 + CD15 + MSPCs obtained from the bone tissue of fetal mice into the renal capsule of adult murine models could foster the formation of ectopic hematopoietic marrow [48]. Thus, the intricate role of MSPCs requires substantial studies in the irradiated murine models to explore their ability to organize the HSC niche [49]. MSCs confined to the perivascular region constituted a significant fraction of CD146 in humans [47] and CXCL12-GFP, Nestin-GFP, leptin receptor, Prx-1-Cre, inducible Mx-1Cre in the murine models could produce the osteoblast cells and foster the generation of several factors to induce the maintenance of HSCs [50]. The CXCL12 abundant reticular (CAR) cells are confined to the sinusoids and they were co-localized with HSCs across bone marrow [51]. For instance, irradiating the mice with CXCL-12 expressing BM cells resulted in the depletion of HSCs consequently causing defects in both adipogenic as well as osteogenic efficacy of BM [5]. Furthermore, the human CD146 + skeletal stem cells can undergo localization adjacent to sinusoids across BM and are involved in fostering substantial amounts of HSC niche factors including SCF, and CXCL-12 [47]. BM stromal cells express the fibroblast activation protein (FAP) and also a phenotypic expression of CXCL-12, SCF, and Sca-1 is abundant in these cells, and involved in modulating the hematopoiesis [52, 53]. For instance, the ablation of BM stromal cells expressing FAP + could cause depression in the BM followed by the eventual occurrence of anemia, hypocellularity, and decline in the osteogenic cells [54, 55]. Future studies are warranted to explore these mechanisms in the BM microenvironment.
Endothelial cells (ECs)
The endothelial cells are confined to the perivascular HSC niche [56] and evidence described the deletion of gp130 cytokine receptors in ECs that could cause hypocellularity of BM and the decline in overall HSCs [57]. Loss of VEGF receptor-2 functions in the murine models by irradiation caused the impairment of regeneration of sinusoidal ECs consequently blocking the LSK stem/progenitor cells and spleen colony-forming cells [20]. The ECs could promote the maintenance of HSC in the culture conditions [58, 59]. E-selectin expressed by ECs is involved in the HSCs maintenance in BM and for instance, blocking the activity of E-selectin could cause a higher quiescence of HSCs and make them more resistant to irradiated ablation [60]. These studies described the crucial functions of ECs in the maintenance of HSC niche but future studies required to explore the role of ECs in regulating the HSCs maintenance in the in vivo conditions. It is crucial to explore the activity of ECs involvement in modulating the vascular permeability in NPY-treated mouse models by examining the HSPC egress.
T reg cell’s role in hematopoiesis
T Regulatory cells (Foxp3 + regulatory T cells) play a crucial role in maintaining the immune system and self-tolerance. These specialized cells, characterized by the expression of transcription factor Foxp3, have been extensively studied for their ability to regulate immune response. However, a gray area in research has been the role of T cells in hematopoiesis and post-transplant reconstitution [61]. In recent times, several experiments have been undertaken to explore the impact of Treg cells on B-cell lymphopoiesis (Fig. 1) and the function of the BM microenvironment [61]. Researchers have analyzed several T-cell-depleted mice models to understand the relation between T cells and HSC [61]. In T-cell-depleted mice models, it was evident that the B cell lymphopoiesis was downregulated in the bone marrow, however, the B cell population was replenished in a compensatory manner by the transfer of affected HSCs or bone marrow cells into T-reg competent recipients [61] (Fig. 2). Intriguingly, B-cell reconstitution was not impaired in both syngeneic and allogeneic transplantation models with Treg-depleted mice as recipients [61].
To further understand the underlying mechanisms, researchers investigated the production of interleukin-7 (IL-7), a growth factor crucial for B-cell lymphopoiesis. It was revealed that the T cell governs the physiological production of IL-7 which is majorly assisted by a subgroup of ICAM1 + perivascular stromal cells [61]. In the event of T cell depletion, IL-7 production by these stromal cells decreased, suggesting a strong physiological influence of T cells on the differentiation of B cells. There exists a crucial, intricate relationship between T reg cells, B cell differentiation, and the production of essential growth factors. IL-7 can modulate cell fate decisions by increasing the expression profiles of BACH2, EBF1, and PAX5 that concomitantly together conferring to the specification or commitment of B cell progenitors. Previous studies described the significant role of HSC transplantation in patients with T-B + SCID and subsequently concluded the functional role of IL-7 receptor signaling in leukemogenesis [62]. Understanding these mechanisms can help to gain valuable insights into T cell biology, physiology, and its implications for clinical applications for modulating the immunological homeostasis among normal individuals and individuals with BM transplants [61].
The impact of Treg cells on immune reconstitution after transplantation is of particular interest. Development of tolerance by T cells is observed in several transplantation models [63]. Co-infusion of donor Treg cells has been shown to ameliorate graft failure in the allogeneic transplants performed in mice models with induced T cell depletion. Treg cells can provide an immune niche to HSCs, helping them to evade host immunity and favoring their survival. Impaired downstream signaling of inflammatory cytokines such as IFN or TNF can confer to dysregulation of HSCs in the BM [64, 65]. These mediators may be crucial in T cell-depleted transplant models. Interestingly, the functional HSC population remained relatively stable in the T-cell-depleted mice model despite the transition of phenotypic HSC into the cell cycle and expansion phase model. However, as a result of T cell depletion, the differentiation of B cells was observed. This indicates that the BM microenvironment aids the B cell differentiation, which was extremely vulnerable to T cell depletion-mediated immune cell activation. This evidence corroborates the role of T cells in regulating the secretion of inflammatory cytokines (which in turn governs HSC quiescence) and BM physiology and function. The HSC and bone marrow environment have susceptibility to host immune cells. The absence of host Treg cells led to increased host-versus-graft BM alloreactivity, resulting in bone marrow aplasia and decreased survival rates. Furthermore, studies suggest that transplant failure is often observed as a result of T cell depletion and has evident manifestations when those mice are subjected to allogenic transplants.
The HSC niche is composed of various essential components, including a cytokine pool which plays a crucial role in HSC progenitor differentiation into lymphoid cell lineages [66]. Perivascular stromal cells, a crucial component of the HSC niche, secrete growth factors such as CXCL12 and IL-7 for B cell differentiation. In vivo T cell-depleted mice models successfully restored donor B-cell reconstitution when administered external IL-7, highlighting that T cells govern IL-7 production. Subsequently, IL-7 has also been used to restore immune response post-transplant in preclinical studies and clinical trials [67, 68].
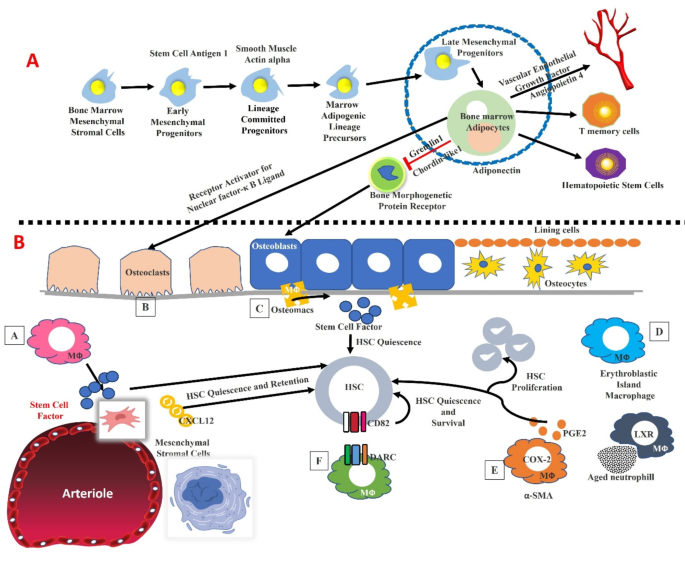
(A). Bone marrow adipocyte lineages and their role in the hematopoietic stem cells and their role on the osteoblasts, and osteocytes across the bone marrow. (B). Heterogeneity of macrophages inside the bone marrow and their role in the hematopoiesis by modulating the functional aspects of niche cells. HSCs dormancy and their maintenance have relied on the macrophages. For instance, the MSCs are modulated by the signals induced by the macrophages. In addition, the macrophages are associated with erythroid progenitors, and factors such as alpha-SMA, DARC, and LXR are expressed by macrophages which could significantly modulate the hematopoiesis by altering HSC function
Administration of IL-7 to syngeneic transplanted mice with adequate T cell levels showed no improvement in the immune reconstitution when compared to their untreated counterparts which proves the satisfactory levels of IL-7 observed in the model and low levels of IL-7 observed only in T cell-depleted mice models [61].
Osteoblast role in hematopoiesis
Recent studies have shed light on the individual contributions of the different hematopoietic lineages, and the evidence thus far suggests that osteoblasts and vasculature are particularly important players in this intricate multiple lineage system [69,70,71]. CD146 + subendothelial cells have been identified as skeletal progenitors that possess an innate ability to restore and reorganize the hematopoietic niche by creating a conducive environment for transplantation in humans [47]. Studies show that the external endosteal surface has abundant vasculature, whose walls exist in close contact with osteoblasts [13, 19].
The trabecular bone lacks endosteal and perivascular/vascular niches, although osteoblasts and vascular cells have different functions. Several reports described the role of osteoblasts within the BM, especially their contribution to maintaining and stabilizing the HSC niche when compared to the osteoblast precursors in different stages which could aid the development of B lymphocytes, which is one of the well-elucidated specific hematopoietic lineages. These studies could provide significant implications for therapeutic interventions, such as BM transplantation and regenerative medicine [72]. Anatomic evidence suggests a longstanding supportive role of osteoblasts in the hematopoiesis within the bone marrow [73, 74]. Osteoblasts do not directly influence HSC function and their maintenance; however, they do govern B cell lineage progenitor activity. This is evident in experiments where osteoblast ablation in adult mice led to the destruction of common lymphoid progenitors (CLPs) whereas B-cell lymphopoiesis was enhanced in cultures enriched for osteoblasts. Cultures enriched for osteoblasts support B lymphopoiesis and ablation of osteoblasts in adult mice acutely depletes common lymphoid progenitors (CLPs) [75, 76]. Gs-alpha knockout osteoblasts are essential for PTH receptor signaling and dramatically reduce pro and pre-B cells which are restored with exogenous IL-7 [77]. Nearly 30% of IL7R + Lineage-bone marrow cells enriched for early lymphoid progenitors are recruited instantaneously towards bone lining cells at the endosteum [15] (Fig. 1).
Macrophages
Macrophages localized in the bone marrow niche are major cell populations that facilitate hematopoiesis by modulating the role of downstream HSCs and MPPs. Macrophages possess an exceptional sensing capacity towards inflammatory markers and they can adjust to the dynamic environment while reducing and/or promoting inflammation. They have a significant impact on HSC function. Therefore, macrophages have a profound impact on hematopoiesis and can be a significant therapeutic target where macrophages are involved in disease pathogenesis. CXCL12 is a chemokine factor that enhances the proliferation and maintenance of B lineage progenitors [78, 79] and CLPs [80, 81]. One of the fundamental processes that macrophages are involved in is the clearance of apoptotic cells through a process known as efferocytosis. This process is essential for the efficient removal of dead or damaged cells. Macrophages facilitate efferocytosis by efficient disposal of neutrophils and activation of the LXR-dependant mechanism. This substantiates the influence of macrophage physiology and function on the homeostatic regulation of blood progenitors. Any dysfunction in circadian rhythms due to aging comorbid with obesity and lifestyle and environmental stress can have profound consequences on the mobilization of HSC and HSPC, which has to be explored through substantial future studies. Inflammatory signaling facilitates HSC and HSPC mobilization into circulation simultaneously with the production and release of granulocytes [82,83,84]. The driving factor for mobilization and granulopoiesis is the G-CSF, a growth factor that facilitates proliferation and differentiation [85,86,87]. The consequence of this event is the downregulation of CXCL12, G-CSF receptor signaling is often not necessary in hematopoietic progenitors themselves [88] (Fig. 1), but macrophages are typically required for the G-CSF-dependent HSC mobilization [89]. G-CSF can induce a dramatic reduction of macrophage levels in the bone marrow but the levels remain relatively unaffected in other tissues like the spleen [90, 91]. This demonstrates that localized reduction of the macrophage population inside the bone marrow could foster the mobilization of HSC. This was consistently observed in a study involving “clodronate-loaded liposome” mediated depletion of macrophages which resulted in HSC mobilization and concomitantly promoted the G-CSF-driven HSC mobilization [92].
The resulting mobilization under the above circumstances accounts for a high number of HSCs in the circulation which suppresses CXCL12, and various HSC retention signals [92, 93], some of which may drive the HSC/HSPC pool to the dormancy [94]. Furthermore, macrophages are a diverse population of cells that play crucial roles in tissue homeostasis, inflammation, and immune responses during aging, and obesity. Along with the mobilization of HSCs, macrophages are required for the HSCs bone marrow engraftment post-transplant [94].
Bone marrow macrophages exhibited radioresistance after irradiation and this resistance persisted for 30 days post-radiation. These tolerant macrophages were typically significant for HSC engraftment and reconstitution post-radiation, which is evident by the ‘reduction of HSC engraftment’ by CD169 expressing macrophage depletion mediated by diphtheria toxin receptor (DTR) [94]. This substantiates the persistence of macrophages post-lethal radiation across BM, as these macrophages can undergo slow clearance or proliferation and also possess self-renewal capacity potentiated by M-CSF. Exogenous M-CSF facilitates the expansion of the host macrophage pool which is demonstrated to have a protective effect on native macrophages and reduction of graft vs. host disease during transplantation [94].
In an independent study, HSC retention and mobilization in bone was potentiated by the depletion of macrophages which consequently resulted in stable hematopoietic chimerism [95]. However, another study provided contrasting evidence that the reduction in HSC engraftment due to macrophage depletion consequently when combined with radiation has led to the development of a proinflammatory environment which resulted in impairment of engraftment [96]. Clod-lip-induced macrophage depletion is not the sole factor for inflammatory response. This inconsistency highlights the important gaps in the interpretation of macrophage depletion studies since they only allow for in vivo modulation of inflammation and immune cells. Clod-lip-mediated macrophage depletion has been widely studied and the underlying mechanism is well established. It essentially involves apoptosis of the phagocytic cell (engulfing liposome) which is triggered by the release of the bisphosphonate intracellularly [97]. However, its deletion in osteoblastic cells led to a reduction in CLPs and other premature lymphoid progenitors in the BM without affecting HSCs [15]. Hence some premature lymphoid progenitors rely on the osteoblast microenvironment rather than the HSC-specific niche. Other lineage-specific microenvironments include the erythroid niche where macrophages are crucial for erythropoietic cell maturation [98]. An in-depth analysis of the erythroid niche is necessary to determine characteristic cellular components to further elucidate the interlink between this niche and existing HSC lymphoid progenitor niches. In vivo, experiments involving induced and conditional alteration or deletion of niche-specific factors and analyzing the manifestations and effects on stem/progenitor cell maintenance provides insights on the stem cell specific-niche and specific progenitor belonging to haematopoietic system, restricted by the precision of available Cre alleles. Thus, gaining an understanding of the cellular and functional differences between these niches is important for advancing our knowledge of haematopoiesis and immune cell development. It opens up new avenues for research into how the bone microenvironment influences the fate and function of different types of blood cells. This knowledge could have implications for developing new strategies to manipulate immune cell production and function, potentially leading to novel therapies for immune-related disorders.
Perivascular cells
Perivascular cells are a crucial niche for modulating the role of adult HSCs. These perivascular cells coexist in an interactive environment with HSCs in adult BM and serve as precursors to mesenchymal stem/stromal cells (MSCs) that foster differentiation into various cell kinds [99], including osteoblasts, adipocytes, and chondrocytes. Pericytes are typically located on the abluminal side of blood vessels; they also maintain significant association with endothelial cells (ECs) and express markers including CD146, NG2, PDGFRβ, α-SMA, Nes, and LepR [99].
However, pericytes are not a homogenous population. Different subsets exhibit distinct immunophenotypes and mesenchymal features, including varying capacities to support hematopoiesis. Both arteriolar and sinusoidal pericytes play crucial roles in regulating HSC behavior, maintenance, and HSC quiescence, and trafficking inside the adult BM via paracrine signaling [99]. Various studies have described hematopoietic-supportive pericyte subpopulations with the aid of diverse markers through murine experiments. Arteriolar pericytes, are marked by upregulated α-SMA and NG2 levels, and they are associated with the maintenance of HSC quiescence and long-term repopulating capacity. Sinusoidal pericytes, often expressing CD146 and PDGFRβ, are implicated in the regulation of HSC mobilization and homing [99]. For instance, Nestin-expressing pericytes have been shown to secrete high levels of CXCL12, a critical chemokine for HSC retention in the BM niche. Similarly, LepR-positive pericytes have been identified as key regulators of HSC maintenance through their support of the sinusoidal microenvironment. These findings suggest that specific pericyte subpopulations create distinct niche environments that differentially influence HSC function [99]. Detailed molecular profiling of pericyte subpopulations will enhance our understanding of their specific roles in HSC regulation. Single-cell RNA sequencing could provide insights into the gene expression profiles and signaling pathways active in different pericyte subsets [99]. Investigating the functional impact of pericyte-HSC interactions using advanced in vivo models and genetic manipulation techniques will clarify the mechanisms through which pericytes influence HSC behavior. Conditional knockout models targeting specific pericyte markers or signaling pathways could elucidate their roles in HSC maintenance and mobilization. Leveraging the knowledge of pericyte subpopulations to engineer BM niches in vitro could have significant implications for HSC transplantation and regenerative medicine. Creating bioengineered niches that mimic the in vivo perivascular environment may improve the expansion and functional maintenance of HSCs ex vivo. Exploring the changes in pericyte function and HSC support with aging or in disease contexts, such as myelodysplastic syndromes or leukemia, will provide insights into how pericyte-HSC interactions are altered in pathological states. This could lead to targeted interventions that restore normal niche function and improve patient outcomes [99].
The rapid growth of the skeletal system during embryonic and postnatal life necessitates a coordinated interplay between cell proliferation, differentiation, mineralization, and the expansion of local vasculature [100, 101]. Several bone-forming cells including chondrocytes and osteoblasts are implicated in this process by releasing VEGF, which stimulates angiogenesis by stimulating VEGF receptors confined on ECs [100,101,102]. Vascular ECs can generate paracrine signaling which could modulate growth as well as regeneration in multiple organs including the skeletal system [103,104,105,106,107]. For instance, osteogenesis is particularly mediated through a specialized capillary EC subtype known as type H; type H-containing capillary ECs are characterized by the expression of CD31/PECAM1 as well as Endomucin (CD31hi Emcn hi) markers. These cells are predominantly located in the metaphysis and endosteum of postnatal long bones [103,104,105,106,107,108]. Type H ECs not only promote angiogenic growth but also release molecular signals that act on osteoprogenitor cells, subsequently coupling angiogenesis with osteogenesis. Conversely, type L ECs, which exhibit lower CD31 and Emcn expression (CD31 lo Emcn lo), together associated with sinusoidal vessel network across BM [103,104,105,106,107,108,109].
Future studies are required to explore research into modulating the VEGF signaling pathway specifically in type H ECs might offer novel approaches to enhance bone healing and repair, particularly in aging populations where bone regeneration is impaired. In addition, identifying and validating biomarkers for type H and type L ECs could facilitate the monitoring of therapeutic outcomes and the development of precision medicine approaches for bone-related diseases. By addressing these future directions, it is possible to develop more effective treatments for bone diseases, enhance bone repair, and improve overall skeletal health [109].
Correlation of arterial and sinusoidal niches in promoting HSC quiescence
Cell cycle quiescence is significant to maintain HSC’s role in BM. Although several stromal cells are proposed as HSC niches, the precise spatial localization of HSCs in the quiescent stage yet remained ambiguous. A study by Yuya Kunisaki et al. 2013 described that quiescent HSCs are typically confined to small arterioles located across endosteal BM. These arterioles are positively ensheathed for NG2 + pericytes which are distinct from LepR + cells. Decline in NG2 + cells could cause enhanced HSC cycling and a decline in the repopulating HSCs on a long-term basis suggesting that arteriolar niches are crucial to maintaining HSC quiescence [3].
Structurally, arterioles and sinusoids are different; these structural differences are reflected in the altered unique transcriptional programs of ECs across arterioles and venules. For instance, arteriolar Nesperi cells and sinusoidal Nesretic cells are uniquely differentiated through their transcriptional activities; for instance, Nesperi cells exhibit a greater enrichment in genes related to cell cycle quiescence as well as for maintaining the HSC niche. Under genotoxic stress, mitotically active Nesretic sinusoidal niche cells are undergoing destruction but quiescent Nesperi arteriolar niche cells exhibit chemoresistance. This dual association of hematopoietic and mesenchymal stem cells with arterioles suggests that these vessels could coordinate hematopoietic and regeneration of stroma [3, 110]. Further investigations have revealed that the interaction between HSCs and their niche is more complex than previously understood. HSCs in periarteriolar niches are mediated in a quiescence state through the influence of NG2 + pericytes, which secrete factors that suppress cell cycling. This is contrasted by the LEPR + perisinusoidal niche, which supports more active HSC states. The dynamics between these niches suggest a sophisticated regulatory mechanism that ensures the preservation and timely activation of HSCs in response to physiological demands. Moreover, the chemoresistance observed in quiescent Nesperi arteriolar niche cells under genotoxic stress highlights a potential therapeutic target for enhancing HSC protection during chemotherapy. Understanding the molecular signals that confer this resistance could lead to strategies to bolster HSC resilience in patients undergoing aggressive treatments. Detailed molecular studies to elucidate the specific signals and pathways involved in maintaining HSC quiescence within arteriolar niches enable the development of therapeutic interventions that can mimic the protective environment of the arteriolar niche to enhance HSC survival during treatments like chemotherapy. Investigating the interactions between different niche cells, including pericytes, ECs, and mesenchymal progenitors is essential to develop a comprehensive map of HSC regulatory networks. By addressing these, it is possible to understand HSC regulation and develop novel therapeutic approaches to enhance hematopoietic health and regeneration [3].
Adipocytes
Bone marrow adipocytes (BMA) are metabolically active and possess abundant lipid reserves, mitochondria, and endoplasmic reticulum. The number of bone marrow adipocytes is variable during growth and development as is influenced by various internal and external factors like osteoporosis, aging, and calorie-deficit diets [111]. Current research indicates that bone marrow adipocytes have an inhibitory effect on hematopoiesis [111, 112]. Caudal vertebrae are composed of adipocytes, a low number of HSCs, and short-term progenitors when compared to the thoracic vertebrae which contain no adipocytes. Moreover, the pharmacological and genetic downregulation of adipogenesis expedites the restoration of hematopoiesis post-irradiation and bone marrow transplant [112]. However, there is no concrete evidence to justify the role and influence of BMAs on HSC and the bone marrow niche. Recent evidence suggests that adipocytes localized in long bones aid restoration of hematopoiesis post-irradiation by providing a crucial HSC survival factor, stem cell factor (SCF) [113]. However, adipocytes localized in tail vertebrates are known to downregulate hematopoiesis [114]. Fatless A-ZIP/F1 mice models when exposed to radiation resulted in the loss of bone marrow cellularity and HSC inside the long bones, but a higher number of HSCs was observed in caudal vertebrae [114]. This stark contrast was associated with the increased vasculature in the tail vertebrae of the mice, which is absent in long bones [114]. The main requirement for restoration of hematopoiesis is an adequate vascularization of bone marrow [20]. Along with SCF, adiponectin and leptin are two other biomolecules produced by adipocytes that could enhance the proliferation of HSC [115, 116].
The rhesus macaque model has been used to validate the relation between BMAs and hematopoiesis in primates. HSPCs reside adjacent to BMAs. Additionally, BMAT (Bone marrow adipocyte tissue)-enriched medium facilitates the HSC proliferation and differentiation ex vivo. A quantitative protein examination of the BMAT-conditioned medium was performed to understand the underlying mechanism. Out of a total of 994 BMAT-derived proteins, including TGFB1, FBLN1, IGFBP2, LGALS1, TIMP1, and C3, were reported to possess the capacity to regulate and increase the differentiation, adhesion, and mobility of HPSC [117]. Of the 994, 430 proteins have a complex framework, possess paracrine function, and have origin from microvesicles or exosomes. It is also essential to understand that BMAT contains cells apart from BMA including macrophages and granulocytes [117]. Thus, the proteins derived from the BMAT could play a regulatory role in HSC function via these immune cells. BMA’s role in leukemia is a subject of debate and is largely lineage-specific. In vitro and in vivo experiments provide evidence that BMAs inhibit T-acute lymphoblastic leukemia (ALL) proliferation in ALL conditions [118].
Further, BMAs, when cocultured with AML blasts, exhibited typically diminished apoptotic effect and higher proliferative effect in acute myeloid leukemia (AML) [119]. AML blasts enhance the lipolysis of BMAs and the resulting fatty acids are transported to AML cells where they undergo β-oxidation. Recent evidence suggests that AML reduces the adipocyte cellularity in the bone marrow and AML xenograft implying that AML selectively influences adipocyte number along with potentiating lipolysis of existing adipocytes [120]. A comprehensive analysis of the global transcriptome of BMSCs isolated from AML or healthy patients reported altered adipogenic differentiation caused by AML [120]. Furthermore, transwell assays were conducted to explore the interlink between BMA reduction and mitigated myelo-erythropoiesis in AML conditions. From this study, it is evident that BMAs can promote the maturation of myeloid and erythropoietic cells. In another study, adipogenesis was activated by GW1929, a PPARγ agonist, which was found to restore hematopoietic maturation in addition to the inhibition of leukemic growth [120].
In conclusion, BMAs are crucial for the normal maturation of myeloid and erythropoietic cells and hence a valid target for therapy to ameliorate bone marrow failure observed in AML (Fig. 1). BMAs are susceptible to changes in homeostatic metabolism and hence need to be researched further to accurately determine their physiology and role in the pathogenesis of diseases like hematological malignancies [2, 121].
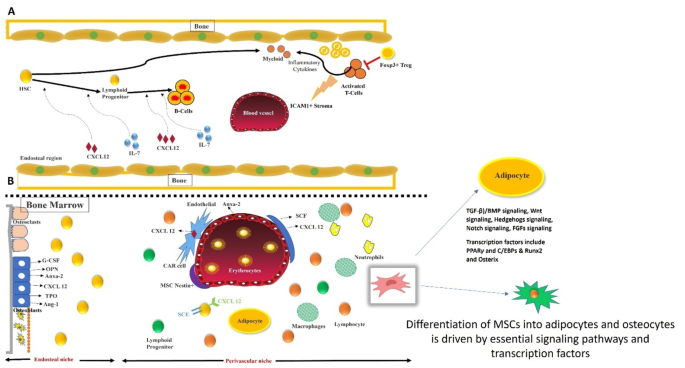
The fine balance between adipogenic and osteogenic differentiation of MSCs is orchestrated by crucial signaling pathways and transcription factors. Signaling pathways such as TGF-β/BMP, Wnt, Hedgehog, Notch, and FGFs play pivotal roles in this regulation. These pathways influence key transcription factors, PPARγ and C/EBPs for adipogenesis, and Runx2 and Osterix for osteogenesis-ensuring a precise differentiation balance. (A). The role of regulatory T cells (Tregs) in the bone marrow environment is significant. Foxp3 + Tregs can influence the activity of cytotoxic T cells and ICAM1 + perivascular cells. Activation of T cells leads to the depletion of Tregs, which in turn reduces UL-7 production by perivascular cells, affecting B cell differentiation from hematopoietic stem cells (HSCs). (B) HSCs within the bone marrow microenvironment are supported by the endosteal and perivascular niches, including osteoclasts, osteoblasts, and other supportive cells. Factors such as G-CSF, osteopontin, annexin-2, thrombopoietin, and angiopoietin-1 regulate HSC functions in lineage commitment. Additionally, osteoblasts, endothelial cells, LepR + perivascular cells, and Nestin + MSCs producing CXCL12 and CAR cells play crucial roles in the homing, self-renewal, and differentiation of HSCs, facilitating hematopoiesis
Primitive hematopoietic cells are confined to the endosteal surface while the progenitors are located centrally within the marrow space. Intravital microscopy experiments were conducted along with HSPCs labeled with significant markers have further confirmed the proximity of HSPCs to the endosteal osteoblasts during the HSPC engraftment conditions where a significant number of mature progenitors were positioned further from osteoblasts [13, 19]. Furthermore, migration of hematopoiesis from fetal liver to bone marrow greatly relies on normal bone formation and turnover during embryonic development. Previous reports suggested that the mice lacking runx2, a key transcription factor for osteoblast differentiation, developed weak, demineralized, or structurally skeletons [122,123,124] which led to compensatory extramedullary [124].
Mice with defective M-CSF exhibit typically reduced levels of osteoclasts which eventually results in osteopetrosis and extramedullary hematopoiesis [125]. In vitro studies have demonstrated the supportive function of stromal cells [126] to the osteoblast lineage to foster hematopoietic cell differentiation [127, 128]. Targeted ablation of osteoblasts was carried out in the mice presenting differentiated osteoblasts with herpes simplex thymidine kinase through ganciclovir administration [76]. This led to a reduction in bone marrow niche and conferred to the extramedullary hematopoiesis, highlighting the role of osteoblasts in supporting hematopoiesis in vivo across the BM [129]. The osteoblast ablation resulted in a downregulation of B-cell lymphopoiesis and erythropoiesis in the BM, eventually mitigating the primitive hematopoietic cell population in bone marrow [75, 129]. Osteoblasts have also been implicated in HSC mobilization, particularly in response to G-CSF [130, 131] (Fig. 2). However, the precise molecular mechanisms and the requirement for direct contact between HSCs and osteoblasts in vivo are still being investigated.
Parathyroid hormone exerts a synergistic effect through osteoblasts on B lymphopoiesis. PTH/PTH-related peptide receptor (PPR) signaling plays a role in osteoblast-mediated B-cell development. Genetic manipulation or alteration of either osteoblast-specific PPR [132] or the BMPR1a receptor [133] results in a higher number of osteoblasts and increased HSC niche [133]. However, a low level of osteoblasts was observed in biglycan knockout mice which is independent of any hematopoietic defect or decrease in HSC [134]. This indicates that osteoblast is not necessarily the sole determining factor of the HSC population [134].
Osteoblasts can regulate functions of various hematopoietic factors including angiopoietin-1 [135], osteopontin [136, 137], thrombopoietin [138], Wnts [139], and extracellular calcium [140]. A recent study suggested that the overexpression of Notch ligand Jagged-1 (Jag1) in osteoblasts by PPR activation directly leads to the increase in the HSC population, thus implicating notch signaling as a factor for hematopoiesis. The overexpression of HSCs can be suppressed by γ-secretase inhibitors [132]. It has also been demonstrated that the deletion of Jag1 Mx1-Cre-mediated in the microenvironment yielded no phenotype [141].
The role of N-cadherin is widely debated among researchers, some who advocate its synergism and importance [19, 133] and others who contest the same [134]. N-cadherin associated with β-catenin/Wnt-signaling has a significant role in the HSCs’ interactions with their niche [142]. Previous studies reported the role of osteoblast N-cadherin mainly cadherin-11 confined to the osteoblasts is upregulated during differentiation. Another study reported that overexpression of c-Myc and Rbm15 can mitigate N-cadherin expression and speculated that the mitigation in the expression of N-cadherin is required for the release from the stem cell niche [143,144,145].
Therefore, while the role of osteoblasts in maintaining the homeostasis of the HSC niche is validated through the studies of in vivo models, the crucial underlying mechanisms remain uncertain. The molecular mechanisms underlying the cross-talk between the osteoblast lineage of the skeletal system and perivascular/vascular cells of the hematopoietic niche is another gray area. Cell-specific ablation of factors will be crucial to addressing several of these issues and bridging the research gap [72].
In vitro cultures of HSCs are not feasible unlike other stem cells sourced from other tissues which can be readily cultured in vitro. This could hinder their therapeutic and transplantation scope, one of them being gene therapy where the transfected HSCs need to be cultured to assess the quality before transplantation. Several factors could influence HSC survival through osteoblasts and the pleiotrophin supplementation promoted HSC survival in vitro [156] and the lack thereof resulted in HSC reduction and dysregulation of haematopoiesis [157]. Pleiotrophin is produced by sinusoidal liver endothelial cells and perivascular stromal cells expressing CXCL12 to promote HSC function [157]. A key slit receptor, Robo4, is expressed by both endothelial cells and HSCs. This receptor governs the HSC localization in bone marrow microenvironment [158, 159]. The slit2 ligand exhibits selective activity towards MSCs and some osteoblast lineage cells. This indicates that both pleiotrophin and Robo4/Slit2 are essential components of the perivascular environment. Evidence also suggests a possible positive or negative influence of Tenascin-C [160], osteopontin [136, 137], and non-canonical Wnts on the HSC population (Tables 1 and 2).
Importance of the different signaling pathways involved with BM niche
As we discussed above, osteoblasts were the first cell type identified to support HSC expansion in vitro, notably via the presentation of granulocyte G-CSF [161]. These cells secrete a variety of proteins, including angiopoietin-1, CXCL12, SCF, and TPO, all of which are pivotal for promoting HSC growth [136, 140, 162]. Additionally, osteopontin and SDF-1α produced by osteoblasts are crucial for the mobilization and egress of HSCs [163]. The perivascular niche, encompassing ECs, perivascular stromal cells, and MSCs, plays an essential role in maintaining HSC physiology near blood vessels. MSCs, which are highly heterogeneous, express markers such as CD146, CXCL12, Nes, and LepR, all of which are vital for HSC survival [16, 47].
Primary ECs obtained from non-hematopoietic organs have been shown to enhance HSCs repopulation in vitro [164]. HSC migration is facilitated by derived from CD31 + ECs as well as E-selectin ligand-1 (ESL-1) in HSPCs. Blocking this interaction results in HSCs becoming quiescent and exerting resistance to the irradiation [60, 165]. Various mature cells in the bone marrow also play significant roles in modifying the niche. Trophic endosteal macrophages, for example, support both osteoblast function and the entire endosteal HSC niche. Their absence can lead to HSC egress into the bloodstream [93, 166]. Non-myelinating Schwann cells regulate HSC pools through the modulation of TGF-β signaling [39, 166]. Expansion of HSPCs is also regulated by the Notch stimulation [167]. Notch signaling plays a dual role: it could foster the morphology development and artery specification subsequently mediates communication among niche cells by enabling Notch receptor expression or ligands. Another critical signaling is the involvement of the canonical Wnt pathway. For instance, overexpression of β-catenin has been associated with a higher hematopoietic cascade [168]. Quiescent LT-HSCs are characterized by the Frizzled 8 expression, which antagonizes Wnt signaling, while Wnt5a maintains HSC quiescence through the inhibition of Wnt3a-mediated canonical Wnt pathway [133]. In addition to these pathways, other signaling mechanisms indirectly influence HSC development, including BMP as well as the Hedgehog pathway. BMP pathway can support the function of spindle-shaped N-cadherin + osteoblasts to control bone marrow niche size and also the fate of HSCs. Sonic Hedgehog is involved in fostering primitive HPCs differentiation and myeloid differentiation [169, 170]. Further, in vivo studies are required for conducting comprehensive in vivo studies to better understand the dynamic interactions within the BM niche and their impact on HSC behavior under various physiological and pathological conditions. As part of regenerative medicine studies, it is crucial to explore the potential of using niche-modifying agents in regenerative medicine to enhance bone and vascular health, thereby improving outcomes in BM transplantation and other hematological therapies.
Adipo-osteogenic differentiation of MSCs and molecular signaling
MSCs undergo a 2-step differentiation involving lineage commitment and maturation, transitioning from multipotent stem cells to lineage-specific progenitors and finally into specialized cell types. Key signaling pathways regulating MSC differentiation include TGFβ/bone morphogenic protein (BMP), Wnt, Hedgehog (Hh), Notch, as well as fibroblast growth factors (FGFs) [171].
The TGFβ/BMP signaling pathway is known to have dual roles in MSC differentiation, affecting both adipogenesis and osteogenesis [172]. This pathway operates through canonical Smad-dependent and non-canonical Smad-independent mechanisms, such as the p38 MAPK pathway [173]. Activation of these pathways regulates the expression of key transcription factors like runt-related gene 2 (Runx2/Cbfa1) [174] and PPARγ, which in turn dictate MSC differentiation [172]. The cytokine composition in the MSC microenvironment is thus crucial for determining lineage commitment.
Wnt signaling can confer osteogenic differentiation but impair adipogenic differentiation [175]. For instance, Wnt3a stimulates osteogenesis via TAZ activation by PP1A-mediated dephosphorylation, and YAP/TAZ can modulate Wnt signaling-induced osteogenesis [176, 177]. Aging-related enhancement in adipocytes is linked to reduced Wnt10b levels. Furthermore, β-catenin loss in the developing mouse uterus mesenchyme switches differentiation towards adipogenesis [178,179,180].
Notch signaling plays a complex role in MSC differentiation. Impairment of Notch pathway promotes autophagy-associated adipogenesis by modulating PTEN-PI3K/AKT/mTOR pathway [181]. Notch also suppresses osteogenesis by inhibiting Wnt/β-catenin signaling but can promote osteogenesis through BMP2 signaling cross-talk [173, 182].
Hedgehog signaling is downregulated during adipogenesis, with decreased Gli expression. Hedgehog pathway actuation inhibits adipogenesis by repressing PPARγ and C/EBPα expression, while Gli inhibition promotes adipogenesis [183]. Conversely, Hedgehog signaling supports osteogenesis [184,185,186] and can interact with BMP signaling to enhance Smad-mediated osteogenesis, highlighting its pro-osteogenic and anti-adipogenic roles [171] (Fig. 2).
Impact of aging on adipo-osteogenic differentiation
Aging is associated with a shift in the differentiation balance of MSCs, leading to increased bone marrow adiposity and decreased osteogenesis [187,188,189]. A previous study identified several molecules linked to age-related osteogenic potential loss, including decreased levels of chloride intracellular channel 1 (CLIC1) as well as prohibitin, and increased levels of LIM and SH3 domain protein 1 (LASP1) and annexin V. Furthermore, aging also increases ROS- mediated oxidative stress, which play significant role in age-induced bone loss as well as differentiation balance through pathways involving FOXO, Wnt, or PPARγ [189,190,191]. For instance, PPARγ, a key transcription factor to modulate adipogenesis, inhibits osteoblast differentiation. Increased PPARγ expression in aged MSCs promotes adipogenesis and inhibits osteogenesis [171]. It is crucial to investigate the interactions between different signaling pathways (e.g., TGFβ/BMP, Wnt, Notch, Hedgehog) to better understand the regulatory networks governing MSC differentiation. Subsequently exploring how microenvironmental factors, such as cytokine composition and mechanical stimuli, influence MSC lineage commitment and differentiation is needed. This could lead to targeted therapies that modulate these factors to promote desired differentiation outcomes. By addressing these research directions, we can better harness the potential of MSCs for regenerative medicine, particularly in the context of aging and bone-related diseases [171].
BM niche regulates stem cell trafficking and age may influence the trafficking in the following signaling pathways
The bone marrow microenvironment is composed of several different kinds of cell types including hematopoietic stem cell progenitors, osteoblasts, immune cells, osteoclasts, and perivascular cells [13, 14]. Irradiation could damage sinusoids across the bone marrow [13, 19, 20] and the arteriolar blood vessels are preserved in the endosteum.
Aging is a highly intricate physiological process associated with significant alterations in tissue-specific changes due to alterations in gene expression and cell composition [192]. In the bone marrow (BM), aging leads to an expansion of the HSC pool, with HSCs exhibiting a biased differentiation toward myeloid progenitors at the expense of lymphoid ones, along with a diminished regenerative potential [35, 193]. Mitosis analysis described that HSCs, and MPPs exhibit a quiescent nature in a steady state whereas the granulocyte-macrophage lineage-restricted progenitors (GMLPs) exhibit a higher proliferation rate [194]. Aging in HSCs is characterized by various intrinsic changes, including loss of cell polarity, heightened Wnt5a non-canonical signaling, disrupted autophagy [195], deregulation of the mitochondrial unfolded protein response, reduced mitochondrial acetylation mediated by SIRT3, and alterations in the epigenome, including increased symmetry of epigenetic division [196,197,198,199,200,201,202,203]. These intrinsic alterations could affect the functions of HSC function irrespective of the BM niche, a phenomenon termed “intrinsic” HSC aging, extensively reviewed elsewhere [204]. Recent investigations into middle-aged BM microenvironments have identified decreased IGF1 levels as a crucial aging-promoting factor affecting both HSCs and niche cells. Restoring IGF1 signaling has been shown to rescue Cdc42 and tubulin polarity, reduce γH2AX focus, and alleviate myeloid differentiation skewing in middle-aged LT-HSCs [205]. Interestingly, fasting-induced decrease of IGF1-dependent stimulation of PKA activity has been identified as a key factor promoting HSC self-renewal, balanced differentiation, stress resistance, and regenerative capacities after chemotherapy in aged mice [206]. This apparent discrepancy might be explained by the different downstream pathways activated by IGF1, with fasting-induced IGF1-mediated effects passing through PKA activation, while aging-associated effects activate the mTOR signaling [205, 206]. This suggests that more research is needed to fully understand the regulation of HSC function by IGF1 during aging. Additionally, aging results in the degeneration and remodeling of various niche compartments, impacting HSC behavior and function on different levels [207].
As the age increases, the alterations occur in the vasculature, marked by the decline in CD31highEndomucin−/low arterioles [147, 151, 208]. Stromal cells inside BM also change, such as the depletion of periarteriolar Osteolectin (+) cells, potentially contributing to the decline in lymphoid progenitors [209]. In contrast, sinusoids remain preserved during aging, potentially explaining the absence of age-related depletion of HSCs in most mouse strains, unlike lymphoid progenitors [208]. The bone marrow’s inflammatory milieu increases during the aging process subsequently contributing to hematopoietic changes. A higher expression of inflammatory factors, including interferons, IL-1β, IL-6, and TNFα by stromal and hematopoietic cells is evident as the age increases in the individuals fostering increased myelopoiesis [4, 150, 210,211,212]. Additionally, alterations in nerve fibers within the bone marrow may play a role in age-related changes in hematopoiesis [146, 213]. Hence, our study specifically focuses on the influence of neural signaling in the hematopoiesis of aging-related hematological malignancies with subsequent need for the development of gene therapies.
Hematopoietic system during aging
With the global population aging, health implications such as cancer, neurodegenerative, and other several ailments contribute to significant public health concerns [214]. Aging pertinent to the hematopoietic system disrupts immunity and homeostasis, increasing the risk of blood malignancies due to impaired HSC function [35, 215,216,217]. Myeloid malignancies are more common with age, whereas lymphoid malignancies are more prevalent in younger individuals [216, 218, 219]. Understanding age-related HSC behavior is crucial for addressing the physiology of the hematopoietic system as age increases. Aging in the hematopoietic system manifests through enhanced myelopoiesis, mitigated ability of adaptive immune functions, and decreased HSC functionality, which are essential for sustaining hematopoiesis. The aging process is characterized by alterations in different HSC subset levels, although the regulatory mechanisms, particularly given HSC heterogeneity, are not fully elucidated. A previous report by Tsu-Yi Su et al. shed light on how aging affects different HSC subset functions, marked by CD49b [219].
Shifts in lineage bias, epigenetic and transcriptional changes with aging
As per the previous reports, both lymphoid-biased as well as myeloid-biased HSC subsets show a shift towards increased myeloid subsets as they age. Additionally, gene expression and regulatory mechanisms in HSCs start to change from the juvenile stage and continue progressively, indicating intrinsic modifications in both cellular and molecular properties due to aging] [219]. Aging is linked to substantial alteration in the transcriptional as well as epigenetic changes that impact the differentiation of HSCs. Previous reports described differentiation-associated gene loci becoming hypermethylated, while self-renewal-associated loci become hypomethylated, with enhanced histone marks activity in aged HSCs [35, 220,221,222]. The study of epigenetic changes in lineage-biased HSC subsets during aging has faced significant limitations, primarily due to difficulties in separating functionally different HSC subsets. As a result, the specific epigenetic modifications that occur in these lineage-biased HSCs have not been thoroughly investigated. Recent advancements in single-cell epigenomics and improved isolation techniques have begun to shed light on these processes. These innovations allow for a more precise dissection of the epigenetic landscape of HSCs, revealing how age-related changes contribute to lineage bias and functional decline. Understanding these mechanisms is crucial for developing targeted therapies to mitigate age-associated hematopoietic dysfunction.
Tsu-Yi Su et al. [219]identified integrin CD49b as a significant marker for differentiating functional subsets among primitive Lineage–Sca-1 + c-Kit+ (LSK) CD48–CD34–CD150hi (CD150hi) HSC compartment [223]. CD49b– HSCs are confined highly to the myeloid-biased cells, whereas CD49b + HSCs predominantly exhibit lymphoid-biased features. Despite transcriptional similarities, these subsets show different profiles of chromatin accessibility, describing epigenetic regulation of these functionally distinct lineage-biased HSCs [223].
Myeloid shift and HSC composition with aging
The increased myeloid output in aging has been attributed to the mitigated efficacy of HSCs to generate lymphoid subsets. Identification of distinct subsets of HSCs describes alterations in the clonal composition of HSCs which could drive enhanced subsets of myeloid-biased cells [215, 216]. Numerous studies on aging HSCs have examined heterogeneous HSC compartments containing a variety of subtypes, making it difficult to draw clear conclusions about age-related changes in specific, highly enriched HSC subsets. Additionally, research on aging in mouse models often contrasts young adult mice (2–4 months old) with older mice (1.5-2 years old). It’s important to note that HSCs transition from a fetal to an adult phenotype approximately one month after birth, during a phase characterized by rapid tissue growth and high self-renewal activity [224, 225]. This developmental stage may contribute to the higher incidence of lymphoid malignancies observed in children, highlighting the need to include the juvenile period in studies of age-related alterations in HSCs [218]. Recent studies utilizing advanced single-cell sequencing techniques and more refined isolation methods have been initiated to address these challenges, offering deeper insights into the epigenetic and functional dynamics of HSCs across different life stages. This enhanced understanding is essential for identifying therapeutic targets to counteract age-related hematopoietic disorders [219].
HSC quiescence and proliferation with age
HSCs are getting more quiescent and undergo minimal proliferation as the age increases [219]. However, the cell cycle pertinent to these aged HSCs is yet unexplored vividly and debated [31, 221, 226], but as per the conclusions given by Tsu-Yi Su et al. juvenile mice typically undergo symmetric self-renewal process whereas aged HSCs could induce the generation of progenitors via symmetric proliferation [224]. Hence, in the aged HSCs, the enhanced production of progenitors aligns with stemness loss and mitigated functionality of HSCs [31, 215,216,217]. Despite the reduction in this functionality, the phenotypic HSC population is enhanced in older age and maintains a larger population size by constraining the cell cycle process. Typically, the CD49b + HSCs were observed to be less quiescent and exhibits higher proliferation rate subsequently associated with minimal engraftment efficacy concluding to explore the CD49b– and CD49b + HSCs ability undergo symmetric or asymmetric division as the age increases; this exploration presenting a significant aspect for future research to uncover HSC heterogeneity as the age of the individuals increases.
Epigenetic regulation of HSC lineage bias
Lineage bias is considered to be a heritable trait [227] suggesting epigenetics may control heterogeneity of HSCs [228]. According to past reports, the functionally distinct HSCs do have identical gene expression patterns but display different epigenetic profiles. Single-cell sequencing/ATAC sequencing described the age-induced gene expression alteration and elucidated that juvenile HSCs have dissimilar molecular identity from fetal HSCs but are transcriptionally identical to adult HSCs. For instance, the chromatic accessibility of HSCs has eventually; the chromatin accessibility typically increased in both HSC subsets, correlating with the loss of SPIB and SPI1 (PU.1) transcription factor binding sites, consistent with the published reduction in PU.1 expression with age [220, 222, 223, 229]. Mitigated SPIB TFBS is vividly observed in adult HSCs and suggests that certain gene expression alterations during aging originate from the juvenile stage typically at the time of the growth restriction process into adulthood [230]. Therefore, these kinds of age-mediated alterations could benefit to identification of epigenetics-associated signatures in age-related hematological malignancies. Subsequently, future studies are required to explore whether targeting chromatin remodeling across these epigenetic regions can reverse or mitigate aging-induced modifications in HSCs.
Subset-specific epigenetic differences
Predominantly, PU.1 levels are critical for myeloid bias; however, PU.1 deficiency in vivo models has been shown to enhance myelopoiesis while blocking lymphopoiesis, thereby increasing the propensity for myeloid leukemia as age increases [231,232,233]. These findings indicate that aging and lineage-biased differentiation may involve shared transcription factors. Furthermore, several other candidate genes have been identified in controlling HSC lineage bias. Specifically, Bcr, Abl1, and Tet1 are typically involved in both myeloid and lymphoid differentiation. In contrast, the role of Kcnn1 in the hematopoietic system remains unexplored. Modulations in the expression or mitigated expression of Bcr, Abl1, and Tet1 have been linked to hematopoietic malignancies, describing a crucial understanding of regulatory functions to modulate normal blood cell lineage differentiation [234,235,236,237,238]. Additional reports are necessary to elucidate the roles of these genes in regulating HSC lineage bias [219].
HSC aging research: Comprehensive genomic analysis of aging HSCs
To explore intrinsic aging mechanisms that compromise somatic stem cell function, we [220] conducted a detailed genomic analysis including histone analysis, and transcriptome/DNA analysis comparing young and aged murine HSCs. According to this analysis, a decrease in TGF-β signaling and disruptions in genes is critical for HSC proliferation or differentiation. Furthermore, aged HSCs displayed broader H3K4me3 peaks across genes associated with HSC self-renewal, alongside enhanced methylation rate of DNA typically at transcription factor binding sites of differentiation-promoting genes and decreased methylation at genes essential for HSC maintenance. These epigenetic modifications collectively reinforce self-renewal while diminishing differentiation, mirroring phenotypic aging in HSCs [220]. Additionally, ribosomal biogenesis is considered to be targeted during aging for modulating the ribosomal protein or RNA genes [220]. Previous reports offer a valuable resource for future epigenomic studies on stem cell aging. The observed epigenetic changes align with documented functional and phenotypic alterations in HSCs, such as increased self-renewal, reduced differentiation potential, and a myeloid-biased differentiation ratio. These modifications, though not directly pathological, create a cellular environment prone to age-related diseases like myelodysplastic syndrome (MDS) and leukemia [220].
Reduced TGF-β signaling in aged HSCs
A previous report described a significant reduction in TGF-β signaling pathways in aged HSCs, corroborating previous reports on aging in cardiac and neural tissues [239, 240]. Differential expression of genes involved in the actuation of ligand and bioavailability include MMP-2 and MMP-9 [241, 242], as well as mitigated levels of Smad6 expression, a gene interfering with Smad2 phosphorylation. These insights describe the necessity for further investigation into TGF-β pathway role in HSC aging.
Ribosomal gene transcription alterations
A report by Deqiang Su et al. [220] revealed significant alterations in ribosomal gene transcription with aging. Despite aged HSCs not being more proliferative than their younger counterparts [35, 36], the increase in ribosomal protein gene transcription suggests enhanced splicing and potential translation efficiency. This finding aligns with studies linking ribosome biogenesis defects to bone marrow failure syndromes and malignancies like Diamond-Blackfan anemia [243]. Given the repeated association of ribosomal biogenesis with aging in various models [244,245,246,247], revisiting its role in mammalian stem cell aging is imperative.
H3K4me3 modifications and aging
Increased breadth of H3K4me3 peaks in aged HSCs, particularly on genes associated with self-renewal, suggests a correlation with functional declines observed in aging HSCs. This phenomenon mirrors observations in C. elegans, where mutations in H3K4 methylation genes extend longevity [248]. H3K4me3 accumulation on genes critical for self-renewal may drive the observed functional impairments in aged HSCs [249, 250].
Linking HSC aging to myeloid malignancies
MDS and AML prevalence increases as the age increases, which is characterized by mitigated differentiation and a myeloid-lineage bias. Aging HSCs acquire changes predisposing them to these malignancies even without mutations. For instance, genes like Dnmt3a and Ezh2, often mutated in MDS and AML, show slight but significant downregulation with age [251, 252]. Furthermore, hypomethylation and upregulation of HSC-specific genes (e.g., Gata2, Hmga2) and hypermethylation of differentiation-related transcription factors (e.g., Pu.1) suggest a predisposition towards malignant transformation. These observations warrant further investigation into the causal relationships between epigenetic changes and hematologic malignancies [251,252,253,254]. Developing novel interventions to modulate HSC function based on these insights holds promise for mitigating age-associated hematopoietic dysfunctions and improving health outcomes. By integrating these detailed genomic analyses and identifying specific future research directions, this discussion offers a comprehensive perspective on the mechanisms driving HSC aging and potential therapeutic strategies [220].
Aging, Diabetes, and obesity-induced BM alterations: hematopoiesis
Transcriptional dysregulation could be one of the factors that can promote aging. Cell cycle regulators [221], upregulated myeloid signatures [221, 255], leukemia-causing genes [35], upregulation of inflammatory signaling through NF-kB, TNF-α [256, 257], and mitigation in the DNA repair pathways [36] are predominantly accompanied by aging. Furthermore, the epigenetic alterations concomitant with transcriptional lesions are significantly observed in HSPCs derived from elderly individuals [220, 258]. NTN1 I is a linchpin molecule actively involved in regulating BMEC-LepR + MSC niche A study by Pradeep Ramalingam et al. 2023 described the regulatory role of NTN1 in BMEC-LepR + MSC niche interactions inside the sinusoids of BM and ameliorate the accumulation of adipocytes [9]. Future studies are warranted to explore the role of BM niche-derived signaling to modulate the role of HSCs in vascular integrity, oxygenation, and adiposity inside the aging BM with hematological malignancies.
Leukemia: Insights into epigenetic regulation and clonal hematopoiesis in aging
Epigenetic regulation plays a crucial role in fostering transcriptional functions in stem cells in aging. Numerous clinical studies have identified gene mutations that could encode epigenetic enzymes in elderly individuals with oligoclonal hematopoiesis or patients with MDS or AML. The significant genes mainly affected are DNMT3A, EZH2, TET2, and SETDB1 [259,260,261]. Although the enzymatic activities of these proteins (DNA methylation/demethylation, H3K27/H3K9 tri-methylation) are well characterized, their specific effects on stem cell function remain unclear. It is elucidated that gene mutations pertinent to DNMT3A, EZH2, TET2, and SETDB1 can induce changes in the epigenetic memory of stem cells; this leads to modulation in transcriptional alterations which in turn fosters the self-renewal ability of mutant cells. Over time, these mutations promote clonal expansion within the bone marrow. Murine model-based reports described that changes in the expression of wild-type or mutate epigenetic writes can modulate the self-renewal process of HSCs [259,260,261,262].
Clonal hematopoiesis is a common phenomenon in older individuals. Initial studies, which examined X-chromosome inactivation patterns in the blood cells of elderly females, suggested that blood cells in older individuals originate from fewer stem cells [263,264,265]. More recent large-scale sequencing studies have confirmed these early observations and revealed that clonal hematopoiesis in healthy individuals increases the risk of developing leukemia, thereby associating it with increased mortality [265]. This raises critical questions about the detrimental nature of clonal hematopoiesis and its underlying mechanisms.
Despite its association with disease, clonal hematopoiesis is present in many healthy elderly individuals without any clinical symptoms [266,267,268]. This suggests that while clonal hematopoiesis can be linked to disease, it may also represent a benign aging process. Mutations in epigenetic genes might be a factor to drive clonal hematopoiesis might not be directly oncogenic but rather increase HSC self-renewal, leading to clonal expansion. When leukemic transformations occur, these actively self-renewing stem cells are more likely to acquire additional mutations, contributing to disease progression. Therefore, it is important to differentiate between mutations that drive benign clonal expansion and those that initiate leukemia.
Despite the established prevalence of clonal hematopoiesis in the elderly, the reports related to clonal stem cell impact on hematopoiesis are limited. A few reports proposed, to describe ranging from clonal succession to stochastic behavior, but no consensus has been reached [269, 270]. This uncertainty arises because clonal descent of blood cells is historically difficult to trace. Early methods involved ex vivo barcoding of purified HSCs, followed by transplantation into recipient mice [271,272,273]. More recent approaches have used in vivo DNA barcoding and fluorescent dyes for clonal marking in transgenic mice and fish [274, 275]. Exploring the roles of candidate genes identified in our ATAC-seq analysis, such as Bcr, Abl1, and Tet1, in regulating HSC lineage bias will be crucial for understanding the regulatory mechanisms pertinent to normal blood lineage differentiation and developing strategies to address hematopoietic malignancies associated with aging.
Understanding the functional impact of epigenetic changes on mature blood cells derived from aged stem cells will provide deeper insights into the links between clonal hematopoiesis and non-hematological diseases. Moreover, reconciling the differences in clonal contribution models and determining the precise number of stem cells contributing to steady-state hematopoiesis at the time of aging will be essential. Enhanced methods for tracing clonal descent in human studies, combined with experimental data from transgenic models, will be vital in addressing these gaps. Finally, translating these findings into clinical strategies to monitor and potentially modulate clonal hematopoiesis could lead to improved management of age-related hematopoietic and cardiovascular diseases [217].
Diabetes
Induces the reduction in the circulating BM-derived stem/progenitor cells and causes severe BM-related complications [10, 276, 277]. Inflammation has a significant role in the derangement of the BM microenvironment in the mouse models of type 1 diabetes as indicated by the presence of proinflammatory cytokines, and other monocyte/macrophage stimulating factors [278]. Changes in the cytokine expression in the long-term T1D conditions are accompanied by higher levels of IGF-1, IGFBP5, and osteoprotegerin and VEGF; concomitantly mitigated gene expression of Bmi1 could induce a decline in the HSPC senescence and fosters impairment of regenerative ability of HSC niche in the sinusoids of BM [10, 279].
Murine models
Disautonomia is associated with diabetes which could affect bone marrow function and damage to the peripheral clock. These events cause damage to the HSC niche during G-CSF-induced HSC mobilization in murine models [280, 281]. Cardiovascular diabetic autonomic neuropathy is an abnormality that can induce mitigated levels of HSPCs with the upregulated expression of src homology & collagen homology domain (p66Shc), which consequently downregulates the expression of sirtuin 1 (Sirt1) [282]. Several in vivo studies delineated the diabetes-induced defects inside the microenvironment of BM including microangiography and neuropathy subsequently impair the stem cell mobilization egress. However, these mechanisms yet should be explored during hematological malignancies comorbid with diabetes.
Comorbid conditions such as obesity, and diabetes could foster proinflammatory conditions which could can induce CHIP-associated pathologies. The relationship between obesity and CHIP was described by Santosh P et al. [12]. In this study, authors elucidated the Ca2+/calcineurin-mediated Nfat and IRG1 signaling in the ob/ob fatty bone marrow recipient mice transplanted with Tet2–/– HSC/Ps which was accompanied by the substantial levels of itaconate [283], a metabolite involved in retrieving TCA cycle and impairs succinate dehydrogenase [284]. In addition, the obesity-mediated alterations in glucose availability induce a higher acetyl-CoA which concomitantly enhances the influx of divalent calcium into the cell. Subsequently, these events could cause activation and translocation of NFAT into the nucleus and result in the activation of lysine acetyltransferase p300 to foster the site-specific regulation of H3K27ac and promote Irg1 upregulated expression and cause accumulation of itaconate. This metabolite bonds to the alpha-ketoglutarate of the TET enzyme and impairs its catalytic activity at the time of DNA demethylation [285, 286]. Santosh P et al. concluded that the transgenic Tet2-/-ob/ob mutant mice and chimeric ob/ob FBM recipient mice transplanted with Tet-/- HSC/Ps induced higher divalent calcium levels [12] and consequently declined the 5-hmC levels in their HSC/Ps; this a vivid role of obesity mediated CHIP-associated hematological malignancies are evident and the novel therapeutic modalities like gene-corrected stem cells may deliver effective clinical outcomes in the aging-associated mutated CHIP populations of BM and future studies are warranted to explore these modalities in clinical sectors.
Impact of atherosclerosis and myocardial infarction (MI) on the HSC niche
Haematopoiesis, the process by which mature immune cells are produced, plays a crucial role in responding to vascular injury, including in atherosclerosis and MI. During these cardiovascular events, infiltrating monocytes release pro-inflammatory cytokines such as TNFα, IL-1β, IL-6 and reactive oxygen species, which amplify local inflammation and promote further leukocyte recruitment. These monocytes also secrete metallopeptidases that contribute to pathogenic remodeling, such as destabilizing the fibrous cap in atherosclerosis and forming infarct scars in MI [287, 288]. In the context of MI, monocyte infiltration occurs in two phases: an initial phase dominated by classical monocytes (Ly6hi CCR2hi in mice; CD14hi CD16 − in humans) that drive inflammation and phagocytosis, followed by a phase where nonclassical monocytes (Ly6Clo CX3CR1hi in mice; CD14lo CD16hi in humans) predominate to facilitate inflammation amelioration and cardiac remodeling. Nonclassical monocytes in this second phase are primarily derived from the conversion of classical monocytes, with minimal recruitment from the periphery [289,290,291,292].
Neutrophils, the most abundant circulating leukocytes, play a significant role in atherogenesis by releasing granules containing pro-inflammatory mediators, proteases, and enzymes that produce reactive oxygen species. These actions promote further leukocyte recruitment and the formation of neutrophil extracellular traps, which contribute to thrombosis. During MI, neutrophils also aid in angiogenesis and macrophage polarization toward an anti-inflammatory state, promoting cardiac tissue healing [290].
Correlation of MI and Clonal Hematopoiesis of Indeterminate Potential (CHIP)
As per the previous reports, the influence of post-MI heart failure on the vasculature of bone marrow elucidated that a significant reduction in type H bone endothelial cells (ECs), typically associated with inflammation due to the release of a higher IL-1β in type H vessels preceding their loss after MI. Blocking generation of IL-1β protected bone vascular niche, suggesting inflammatory signaling as the primary cause of type H vasculature reduction post-MI. It is speculated that IL-1β activation in CD31hiEMCNhi ECs can confer to pyroptosis and subsequently damage the endothelium of H vessels subsequently leading to age-induced damage to bone endothelium which further results in damage to hematopoiesis and activity of HSCs [151, 293,294,295].
Given that MI could foster myeloid skewing and increase the number of LT-HSCs, it is plausible that MI could cause speedy HSC expansion with CHIP-driver mutations. In reality, individuals with ischemic heart disease are associated with a greater chance of incidence of CHIP-driver mutations when compared to age-matched cohorts [296, 297]. An exploratory analysis from the CANTOS trial revealed that patients with CHIP mutations (e.g., TET2) experienced lower mortality than those without CHIP mutations [298].
To further elucidate the mechanisms underlying the interaction between cardiovascular diseases and the HSC niche, a detailed investigation into how cytokines and DAMPs interact with the NLRP3 inflammasome and other inflammatory pathways will be essential in understanding the progression from cardiovascular injury to hematopoietic dysregulation. Research should focus on how specific HSC subsets contribute to hematopoiesis during cardiovascular disease and aging. Investigating the differential adhesion and migration properties of these subsets could reveal targets for therapeutic intervention. On the other hand, conducting longitudinal studies using advanced clonal tracking techniques will help determine the stability and contribution of various HSC clones over time, particularly in the context of cardiovascular diseases. Expanding clinical trials to test the efficacy of anti-inflammatory treatments, like canakinumab, in broader populations and different stages of cardiovascular disease could provide new insights into managing CHIP and related hematopoietic anomalies. Furthermore, comprehensive profiling of genetic and epigenetic changes in HSCs from patients with cardiovascular diseases will help identify biomarkers for early detection and potential targets for personalized therapies. By addressing these areas, we can better understand and potentially mitigate the adverse effects of cardiovascular diseases on the hematopoietic system, improving outcomes for affected individuals [299].
Bi-directional impact of clonal hematopoiesis on the aging bone marrow niche
Understanding the bi-directional influences of CHIP on the aging bone marrow niche is crucial, as this interaction may elucidate the early changes that precede myeloid disease manifestation and potentially reveal targets for early intervention.
Inflammaging and Niche remodeling
A key concept in CHIP-related research is the acceleration of inflammaging, a chronic, low-grade inflammation associated with aging, which exacerbates BM niche remodeling beyond what is observed in normal aging. Genetic subtypes of CHIP, such as DNMT3A, TET2, and JAK2 mutations [300], have been linked to enhanced inflammatory responses. CHIP carriers exhibit typically enhanced proinflammatory cytokines, such as IL-6, IL-8, TNF, IL-1β, and IL-18 [268, 301, 302]. Gene-specific analyses highlight that TET2 mutations are associated with enhanced IL-1β, while JAK2 as well as SF3B1 mutations correlate with higher IL-18 levels [268, 301, 302]. This elucidates the necessity for gene-specific studies to understand the distinct inflammatory profiles induced by different CHIP mutations.
CHIP mutations also appear to influence bone structure, particularly in DNMT3A-mutant carriers, who are prone to osteoporosis. Proinflammatory cytokines from DNMT3A-deficient macrophages, such as IL-20, enhance osteoclastogenesis, leading to increased bone resorption and reduced bone mass, thereby deteriorating the endosteal niche [268, 301, 302]. Similarly, Tet2 inactivation in mice results in decreased bone mass, although the effects are less severe [268, 301, 302]. The involvement of growth factors like TGF-β and BMPs in bone resorption and MDS progression further illustrates the complex interplay between CHIP and bone health [303, 304]. Emerging evidence suggests that CHIP mutations may impact MSC function and differentiation capacity. For instance, DNMT3A-mutant HSPCs induce MSC senescence via IL-6 production, which may result in clonal expansion and myeloid skewing [305,306,307]. Another condition including sympathetic neuropathy induced through mutant cells could also impair MSCs’ HSC-supportive function, highlighting a potential mechanism for CHIP-induced niche remodeling [305,306,307]. CHIP mutations may also promote vascular niche remodeling. Tet2-deficient immune cells, particularly myeloid cells, have been shown to enhance angiogenesis through increased S100A8/A9 secretion in a lung cancer model [308]. This pro-angiogenic and inflammatory environment likely increases BM vascularization and perivascular permeability, activating transcriptional activities pertinent to inflammation inside stromal cells as well as endothelial cells in BM during aging [267, 309].
Selective advantage and clonal expansion
Aging and adipocyte-enriched BM is characterized by prolonged TNF signaling, which confers a selective advantage to DNMT3A- and TET2-mutant clones, facilitating their expansion [12, 310]. In this case, IL-1R pathway is reported in driving Tet2+/− CHIP progression with aging [311, 312]. Additionally, TET2 mutations may impair natural killer (NK) cell-mediated immune surveillance, further promoting the persistence of malignant clones [313].
The interplay between CHIP and the aging BM niche involves a continuous bi-directional inflammatory loop. Mutant HSPCs not only adapt to but also drive the remodeling of both the endosteal and vascular niches, creating a microenvironment that favors clonal expansion. This intricate relationship between intrinsic HSPC mutations and extrinsic niche factors elucidates the complexity of CHIP and its progression to hematologic malignancies [314]. Further studies should explore the molecular pathways through which CHIP mutations induce niche remodeling and inflammaging for identifying potential therapeutic targets to modulate the BM niche and inhibit CHIP progression. Developing strategies for early intervention in CHIP carriers is essential to prevent the transition to overt myeloid malignancies. In addition, it is advantageous to conduct gene-specific analyses to better understand the distinct inflammatory and remodeling responses induced by different CHIP mutations. By elucidating these mechanisms, we can develop targeted therapies to mitigate the adverse effects of CHIP on the aging BM niche and improve outcomes for individuals at risk of hematologic diseases [314].
Conclusions and future acknowledgments
Aging, obesity, and diabetes can cause significant alterations in the bone marrow. The factors concomitantly generated by these conditions can influence signaling across the BM, subsequently causing changes in hematopoiesis. These alterations can disrupt the normal function and regulation of HSCs and HSPCs, leading to an increased risk of hematological disorders and malignancies. Future studies are warranted to explore the following areas: Influence of autonomic signaling: Investigate how autonomic signaling affects the regulation of hematopoiesis, particularly in the context of aging, obesity, and diabetes. Understanding these mechanisms could reveal novel therapeutic targets to maintain healthy hematopoiesis. Age-related changes in hematopoiesis: Delve deeper into how aging-induced epigenetic influences hematopoietic processes at the cellular and molecular levels. Identifying specific age-related changes could help in developing strategies to counteract the adverse effects of aging on the hematopoietic system. Obesity-related clonal hematopoiesis: Study the impact of obesity on clonal hematopoiesis and its role in promoting hematological malignancies. Research in this area could lead to interventions that mitigate the risk of cancer in obese individuals by targeting the pathways involved in clonal expansion and mutation accumulation. Addressing these areas in future research could significantly advance our understanding of the complex interplay between systemic conditions like aging, obesity, diabetes, and hematopoietic regulation. This knowledge would be instrumental in developing novel therapeutic strategies to prevent and treat hematological malignancies associated with these conditions.
Abbreviations
- BM:
-
Bone marrow
- Tet2:
-
Tet methylcytosine dioxygenase 2
- Dnmt3a:
-
DNA (cytosine-5)-methyltransferase 3 A
- Asxl1:
-
ASXL transcriptional regulator 1
- Jak2:
-
Janus kinase 2
- CXCL12:
-
C-X-C motif chemokine ligand 12
- SCF:
-
Stem cell factor
- CFC-F:
-
Bone marrow fibroblastoid colony-forming cells
- VEGF:
-
Vascular endothelial growth factor
- HSPCs:
-
hematopoietic stem and progenitor cells
- FOXP3:
-
forkhead box P3
- ICAM-1:
-
Intercellular adhesion molecule-1
- BACH2:
-
B-cell-specific transcription repressor
- EBF1:
-
early B cell factor-1
- PAX5:
-
Paired Box (PAX) transcription factor 5
- SCID:
-
severe combined immunodeficiency
- IFN:
-
interferon
- TNF:
-
tumor necrosis factor
- Runx2:
-
Runt-related transcription factor-2
- M-CSF:
-
Macrophage colony-stimulating factor
- γ-H2AX:
-
Gamma-H2AX
- LT-HSCs:
-
Multipotent long-term HSCs
- CDC42:
-
Cell Division Cycle 42
- Slit2:
-
Slit guidance ligand 2
- IGF-1:
-
insulin-like growth factor-1
- SP1:
-
Specificity protein-1
- CXCR4:
-
chemokine receptor 4
- β-3-ADR:
-
Beta-3-adrenergic receptors
- SMAD:
-
Suppressor of Mothers against Decapentaplegic
- TAC:
-
tachykinin
- NK:
-
neurokinin
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- MIP-1 alpha:
-
macrophage inhibitory protein-1 alpha
- SNS:
-
Sympathetic nervous system
- DPP4:
-
Dipeptidyl peptidase 4
- MMP:
-
metalloproteinase
- BMEC:
-
Bone Marrow endothelial cells
- NTN1:
-
Netrin-1
- IGFBP-5:
-
Insulin-like growth factor binding protein
- AML:
-
Acute myeloid Leukaemia
References
Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. <ArticleTitle Language=“En”>Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regenerative Med. 2019;4(1):22.
Wang L, Zhang H, Wang S, Chen X, Su J. Bone marrow adipocytes: a critical player in the bone marrow microenvironment. Front Cell Dev Biology. 2021;9:770705.
Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–43.
Ho Y-H, Del Toro R, Rivera-Torres J, Rak J, Korn C, García-García A, Macías D, González-Gómez C, Del Monte A, Wittner M. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell. 2019;25(3):407–18. e406.
Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3):387–99.
Méndez-Ferrer S. Molecular interactome between HSCs and their niches. Blood. J Am Soc Hematol. 2019;134(15):1197–8.
Mende N, Jolly A, Percin GI, Günther M, Rostovskaya M, Krishnan SM, Oostendorp RA, Dahl A, Anastassiadis K, Höfer T. Prospective isolation of nonhematopoietic cells of the niche and their differential molecular interactions with HSCs. Blood J Am Soc Hematol. 2019;134(15):1214–26.
Fielding C, Méndez-Ferrer S. Neuronal regulation of bone marrow stem cell niches. F1000Research 2020, 9.
Ramalingam P, Gutkin MC, Poulos MG, Tillery T, Doughty C, Winiarski A, Freire AG, Rafii S, Redmond D, Butler JM. Restoring bone marrow niche function rejuvenates aged hematopoietic stem cells by reactivating the DNA Damage Response. Nat Commun. 2023;14(1):2018.
Fadini GP, Ferraro F, Quaini F, Asahara T, Madeddu P. Concise review: diabetes, the bone marrow niche, and impaired vascular regeneration. Stem cells translational Med. 2014;3(8):949–57.
Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, van Buul JD, van Alphen FP, Graiani G, Spinetti G. Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol. 2010;30(3):498–508.
Pasupuleti SK, Ramdas B, Burns SS, Palam LR, Kanumuri R, Kumar R, Pandhiri TR, Dave UP, Yellapu NK, Zhou X. Obesity-induced inflammation exacerbates clonal hematopoiesis. J Clin Investig 2023, 133(11).
Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Côté D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457(7225):92–6.
Sipkins DA, Wei X, Wu JW, Runnels JM, Côté D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435(7044):969–73.
Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5.
Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62.
Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, MacArthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34.
Zhou X, Von Der Mark K, Henry S, Norton W, Adams H, De Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014;10(12):e1004820.
Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, Grindley JC. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457(7225):97–101.
Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp H-G, Shido K, Petit I, Yanger K. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4(3):263–74.
Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 2011;175(2):145–9.
Cheng T, Rodrigues N, Shen H, Yang Y-g, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804–8.
Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J, Li L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20(11):1321–6.
Ye M, Zhang H, Amabile G, Yang H, Staber PB, Zhang P, Levantini E, Alberich-Jordà M, Zhang J, Kawasaki A. C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence. Nat Cell Biol. 2013;15(4):385–94.
Lee JY, Hong S-H. Hematopoietic stem cells and their roles in tissue regeneration. Int J stem cells. 2020;13(1):1–12.
Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant C, Eshkind L, Bockamp E. Hematopoietic Stem Cells Reversibly Switch from Dormancy to Self-Renewal during Homeostasis and Repairvol 135, pg 1118, (2008). Cell 2009, 138(1):209–209.
Harrison DE. Normal production of erythrocytes by mouse marrow continuous for 73 months. Proceedings of the National Academy of Sciences 1973, 70(11):3184–3188.
Yui J, Chiu C-P, Lansdorp PM. Telomerase activity in candidate stem cells from fetal liver and adult bone marrow. Blood J Am Soc Hematol. 1998;91(9):3255–62.
Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3(4):329–33.
Marley D, Goldman G. Evidence for a continuous decline in haemopoietic cell function from birth: application to evaluating bone marrow failure in children. Br J Haematol. 1999;106(1):162–6.
Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2(9):1011–6.
Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106(4):1479–87.
Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192(9):1273–80.
Kim M, MOON HB, Spangrude GJ. Major age-related changes of mouse hematopoietic stem/progenitor cells. Ann N Y Acad Sci. 2003;996(1):195–208.
Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci. 2005;102(26):9194–9.
Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201.
Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447(7145):686–90.
Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–9.
Muller-Sieburg CE, Cho RH, Karlsson L, Huang J-F, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103(11):4111–8.
Muller-Sieburg CE, Sieburg HB. Clonal diversity of the stem cell compartment. Curr Opin Hematol. 2006;13(4):243–8.
Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee S-J, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1(2):218–29.
Graf T. Two lineages of hematopoietic stem cells identified by differences in lineage priming. In: Experimental Hematology: 2007: ELSEVIER SCIENCE INC 360 PARK AVE SOUTH, NEW YORK, NY 10010 – 1710 USA; 2007: 1–2.
Muller-Sieburg CE, Cho RH, Thoman M, Adkins B, Sieburg HB. Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood J Am Soc Hematol. 2002;100(4):1302–9.
Cho RH, Müller-Sieburg CE. High frequency of long-term culture-initiating cells retain in vivo repopulation and self-renewal capacity. Exp Hematol. 2000;28(9):1080–6.
Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood J Am Soc Hematol. 2008;111(12):5553–61.
Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285–316.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36.
Chan CK, Chen C-C, Luppen CA, Kim J-B, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457(7228):490–4.
Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303–20.
Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–21.
Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88.
Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–96.
Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, Frenette PS. PDGFRα and CD51 mark human nestin + sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7):1351–67.
Tran E, Chinnasamy D, Yu Z, Morgan RA, Lee C-CR, Restifo NP, Rosenberg SA. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210(6):1125–35.
Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, Espeli M, Kraman M, McKenna B, Wells RJ. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med. 2013;210(6):1137–51.
Kiel MJ, Yilmaz ÖH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21.
Yao L, Yokota T, Xia L, Kincade PW, McEver RP. Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood. 2005;106(13):4093–101.
Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6(3):251–64.
Kobayashi H, Butler JM, O’donnell R, Kobayashi M, Ding B-S, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12(11):1046–56.
Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Lévesque J-P. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18(11):1651–7.
Pierini A, Nishikii H, Baker J, Kimura T, Kwon H-S, Pan Y, Chen Y, Alvarez M, Strober W, Velardi A. Foxp3 + regulatory T cells maintain the bone marrow microenvironment for B cell lymphopoiesis. Nat Commun. 2017;8(1):15068.
Kaiser FM, Janowska I, Menafra R, de Gier M, Korzhenevich J, Pico-Knijnenburg I, Khatri I, Schulz A, Kuijpers TW, Lankester AC. IL-7 receptor signaling drives human B-cell progenitor differentiation and expansion. Blood 2023.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87.
Pronk CJ, Veiby OP, Bryder D, Jacobsen SEW. Tumor necrosis factor restricts hematopoietic stem cell activity in mice: involvement of two distinct receptors. J Exp Med. 2011;208(8):1563–70.
Mizutani T, Tsuji K, Ebihara Y, Taki S, Ohba Y, Taniguchi T, Honda K. Homeostatic erythropoiesis by the transcription factor IRF2 through attenuation of type I interferon signaling. Exp Hematol. 2008;36(3):255–64.
Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388(6638):133–4.
Dulude G, Brochu S, Fontaine P, Baron C, Gyger M, Roy D, Perreault C. Thymic and extrathymic differentiation and expansion of T lymphocytes following bone marrow transplantation in irradiated recipients. Exp Hematol. 1997;25(9):992–1004.
Bolotin E, Smogorzewska M, Smith S, Widmer M, Weinberg K. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. 1996.
Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, Moulder G, Barstead R, Wickens M, Kimble J. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature. 2002;417(6889):660–3.
Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. cell 2000, 100(1):157–168.
Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8(4):290–301.
Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24(5):759.
Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46(1):65–72.
Gong JK. Endosteal marrow: a rich source of hematopoietic stem cells. Science. 1978;199(4336):1443–5.
Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–12.
Visnjic D, Kalajzic I, Gronowicz G, Aguila H, Clark S, Lichtler A, Rowe D. Conditional ablation of the osteoblast lineage in Col2. 3∆tk transgenic mice. J Bone Miner Res. 2001;16(12):2222–31.
Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, Scadden DT, Kronenberg HM. Osteoblastic regulation of B lymphopoiesis is mediated by Gsα-dependent signaling pathways. Proceedings of the National Academy of Sciences 2008, 105(44):16976–16981.
Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S-i, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–8.
Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci. 1994;91(6):2305–9.
Nie Y, Han Y-C, Zou Y-R. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205(4):777–83.
Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB. Physiological recirculation of hematopoietic stem and progenitor cells through blood, lymph and extramedullary tissues. Cell. 2007;131(5):994.
Singh P, Hu P, Hoggatt J, Moh A, Pelus LM. Expansion of bone marrow neutrophils following G-CSF administration in mice results in osteolineage cell apoptosis and mobilization of hematopoietic stem and progenitor cells. Leukemia. 2012;26(11):2375–83.
Cain DW, Snowden PB, Sempowski GD, Kelsoe G. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS ONE. 2011;6(5):e19957.
Lévesque J-P, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Investig. 2003;111(2):187–96.
Aiuti A, Webb I, Bleul C, Springer T, Gutierrez-Ramos J. The chemokine SDF-1 is a chemoattractant for human CD34 + hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34 + progenitors to peripheral blood. J Exp Med. 1997;185(1):111–20.
Bendall LJ, Bradstock KF. G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014;25(4):355–67.
Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, Levesque J-P, Chappel J, Ross FP, Link DC. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020–7.
Liu F, Poursine-Laurent J, Link DC. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood. J Am Soc Hematol. 2000;95(10):3025–31.
Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208(2):251–60.
Jacobsen RN, Forristal CE, Raggatt LJ, Nowlan B, Barbier V, Kaur S, van Rooijen N, Winkler IG, Pettit AR, Levesque J-P. Mobilization with granulocyte colony-stimulating factor blocks medullar erythropoiesis by depleting F4/80 + VCAM1 + CD169 + ER-HR3 + Ly6G + erythroid island macrophages in the mouse. Exp Hematol. 2014;42(7):547–61. e544.
Winkler I, Pettit A, Raggatt L, Jacobsen R, Forristal C, Barbier V, Nowlan B, Cisterne A, Bendall L, Sims N. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26(7):1594–601.
Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, Van Rooijen N. Bone marrow CD169 + macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–71.
Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood J Am Soc Hematol. 2010;116(23):4815–28.
Hashimoto D, Chow A, Greter M, Saenger Y, Kwan W-H, Leboeuf M, Ginhoux F, Ochando JC, Kunisaki Y, van Rooijen N. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J Exp Med. 2011;208(5):1069–82.
Li Z, Xu X, Feng X, Murphy PM. The macrophage-depleting agent clodronate promotes durable hematopoietic chimerism and donor-specific skin allograft tolerance in mice. Sci Rep. 2016;6(1):22143.
Kaur S, Raggatt LJ, Millard SM, Wu AC, Batoon L, Jacobsen RN, Winkler IG, MacDonald KP, Perkins AC, Hume DA. Self-repopulating recipient bone marrow resident macrophages promote long-term hematopoietic stem cell engraftment. Blood J Am Soc Hematol. 2018;132(7):735–49.
Lehenkari PP, Kellinsalmi M, Näpänkangas JP, Ylitalo KV, Mönkkönen J, Rogers MJ, Azhayev A, Väänänen HK, Hassinen IE. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002;61(5):1255–62.
Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, Pinho S, Leboeuf M, Noizat C, Van Rooijen N. CD169 + macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19(4):429–36.
Da Bandeira DS, Casamitjana J, Crisan M. Pericytes, integral components of adult hematopoietic stem cell niches. Pharmacol Ther. 2017;171:104–13.
Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 2013;5(1):a008334.
Eshkar-Oren I, Viukov SV, Salameh S, Krief S, Oh C-d, Akiyama H, Gerber H-P, Ferrara N, Zelzer E. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. 2009.
Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, Bouillon R, Carmeliet G. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111(1–2):61–73.
Ding B-S, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505(7481):97–102.
Ding B-S, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468(7321):310–5.
Ding B-S, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147(3):539–53.
Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507(7492):376–80.
Hu J, Srivastava K, Wieland M, Runge A, Mogler C, Besemfelder E, Terhardt D, Vogel MJ, Cao L, Korn C. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343(6169):416–9.
Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–8.
Langen UH, Pitulescu ME, Kim JM, Enriquez-Gasca R, Sivaraj KK, Kusumbe AP, Singh A, Di Russo J, Bixel MG, Zhou B. Cell–matrix signals specify bone endothelial cells during developmental osteogenesis. Nat Cell Biol. 2017;19(3):189–201.
Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31(1):285–316.
Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow adipose tissue: trimming the fat. Trends Endocrinol Metabolism. 2016;27(6):392–403.
Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–63.
Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13(1):102–16.
Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19(8):891–903.
DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, Sankar U, Reya T. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol. 2007;178(6):3511–20.
Poloni A, Maurizi G, Serrani F, Mancini S, Zingaretti MC, Frontini A, Cinti S, Olivieri A, Leoni P. Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol. 2013;41(6):558–66. e552.
Robino JJ, Pamir N, Rosario S, Crawford LB, Burwitz BJ, Roberts CT Jr, Kurre P, Varlamov O. Spatial and biochemical interactions between bone marrow adipose tissue and hematopoietic stem and progenitor cells in rhesus macaques. Bone. 2020;133:115248.
Cahu X, Calvo J, Poglio S, Prade N, Colsch B, Arcangeli M-L, Leblanc T, Petit A, Baleydier F, Baruchel A. Bone marrow sites differently imprint dormancy and chemoresistance to T-cell acute lymphoblastic leukemia. Blood Adv. 2017;1(20):1760–72.
Shafat MS, Oellerich T, Mohr S, Robinson SD, Edwards DR, Marlein CR, Piddock RE, Fenech M, Zaitseva L, Abdul-Aziz A. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood J Am Soc Hematol. 2017;129(10):1320–32.
Boyd AL, Reid JC, Salci KR, Aslostovar L, Benoit YD, Shapovalova Z, Nakanishi M, Porras DP, Almakadi M, Campbell CJ. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat Cell Biol. 2017;19(11):1336–47.
Zinngrebe J, Debatin K-M, Fischer-Posovszky P. Adipocytes in hematopoiesis and acute leukemia: friends, enemies, or innocent bystanders? Leukemia. 2020;34(9):2305–16.
Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89(5):765–71.
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson R, Gao Y-H, Inada M. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–64.
Deguchi K, Yagi H, Inada M, Yoshizaki K, Kishimoto T, Komori T. Excessive extramedullary hematopoiesis in Cbfa1-deficient mice with a congenital lack of bone marrow. Biochem Biophys Res Commun. 1999;255(2):352–9.
Yoshida H, Hayashi S-I, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S-I. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345(6274):442–4.
Sutherland HJ, Lansdorp PM, Henkelman DH, Eaves AC, Eaves CJ. Functional characterization of individual human hematopoietic stem cells cultured at limiting dilution on supportive marrow stromal layers. Proc Natl Acad Sci. 1990;87(9):3584–8.
Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. 1996.
Taichman RS, Emerson SG. The role of osteoblasts in the hematopoietic microenvironment. Stem Cells. 1998;16(1):7–15.
Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–64.
Katayama Y, Battista M, Kao W-M, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–21.
Mayack SR, Wagers AJ. Osteolineage niche cells initiate hematopoietic stem cell mobilization. Blood J Am Soc Hematol. 2008;112(3):519–31.
Calvi L, Adams G, Weibrecht K, Weber J, Olson D, Knight M, Martin R, Schipani E, Divieti P, Bringhurst FR. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6.
Zhang J, Niu C, Ye L, Huang H, He X, Tong W-G, Ross J, Haug J, Johnson T, Feng JQ. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41.
Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1(2):204–17.
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61.
Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201(11):1781–91.
Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106(4):1232–9.
Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1(6):685–97.
Fleming HE, Janzen V, Celso CL, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2(3):274–83.
Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603.
Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105(6):2340–2.
Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106(1):41–53.
Niu C, Zhang J, Breslin P, Onciu M, Ma Z, Morris SW. c-Myc is a target of RNA-binding motif protein 15 in the regulation of adult hematopoietic stem cell and megakaryocyte development. Blood. J Am Soc Hematol. 2009;114(10):2087–96.
Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, Pasche A-C, Knabenhans C, MacDonald HR. Trumpp A: c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18(22):2747–63.
Wein F, Pietsch L, Saffrich R, Wuchter P, Walenda T, Bork S, Horn P, Diehlmann A, Eckstein V, Ho AD. N-cadherin is expressed on human hematopoietic progenitor cells and mediates interaction with human mesenchymal stromal cells. Stem cell Res. 2010;4(2):129–39.
Maryanovich M, Zahalka AH, Pierce H, Pinho S, Nakahara F, Asada N, Wei Q, Wang X, Ciero P, Xu J. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med. 2018;24(6):782–91.
Kusumbe AP, Ramasamy SK, Itkin T, Mäe MA, Langen UH, Betsholtz C, Lapidot T, Adams RH. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532(7599):380–4.
Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank A-M, Bocian C, Woelk L, Fan H, Logan DW, Schürmann A. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6):771–84. e776.
Guidi N, Sacma M, Ständker L, Soller K, Marka G, Eiwen K, Weiss JM, Kirchhoff F, Weil T, Cancelas JA. Osteopontin attenuates aging-associated phenotypes of hematopoietic stem cells. EMBO J. 2017;36(7):840–53.
Ergen AV, Boles NC, Goodell MA. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood J Am Soc Hematol. 2012;119(11):2500–9.
Poulos MG, Ramalingam P, Gutkin MC, Llanos P, Gilleran K, Rabbany SY, Butler JM. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J Clin Investig. 2017;127(11):4163–78.
Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences 2013, 110(41):16574–16579.
Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–8.
Jin J, Wang X, Wang Q, Guo X, Cao J, Zhang X, Zhu T, Zhang D, Wang W, Wang J. Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS ONE. 2013;8(9):e74497.
Hasan S, Johnson NB, Mosier MJ, Shankar R, Conrad P, Szilagyi A, Gamelli RL, Muthumalaiappan K. Myelo-erythroid commitment after burn injury is under β-adrenergic control via MafB regulation. Am J Physiology-Cell Physiol. 2017;312(3):C286–301.
Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, Chi J-T, Salter AB, Lento WE, Reya T. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16(4):475–82.
Himburg HA, Harris JR, Ito T, Daher P, Russell JL, Quarmyne M, Doan PL, Helms K, Nakamura M, Fixsen E. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012;2(4):964–75.
Smith-Berdan S, Nguyen A, Hassanein D, Zimmer M, Ugarte F, Ciriza J, Li D, García-Ojeda ME, Hinck L, Forsberg EC. Robo4 cooperates with CXCR4 to specify hematopoietic stem cell localization to bone marrow niches. Cell Stem Cell. 2011;8(1):72–83.
Smith-Berdan S, Schepers K, Ly A, Passegué E, Forsberg EC. Dynamic expression of the Robo ligand Slit2 in bone marrow cell populations. Cell Cycle. 2012;11(4):675–82.
Nakamura-Ishizu A, Okuno Y, Omatsu Y, Okabe K, Morimoto J, Uede T, Nagasawa T, Suda T, Kubota Y. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood J Am Soc Hematol. 2012;119(23):5429–37.
Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179(5):1677–82.
Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–64.
Mangialardi G, Cordaro A, Madeddu P. The bone marrow pericyte: an orchestrator of vascular niche. Regen Med. 2016;11(8):883–95.
Li W, Johnson SA, Shelley WC, Yoder MC. Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Exp Hematol. 2004;32(12):1226–37.
Sreeramkumar V, Leiva M, Stadtmann A, Pitaval C, Ortega-Rodríguez I, Wild MK, Lee B, Zarbock A, Hidalgo A. Coordinated and unique functions of the E-selectin ligand ESL-1 during inflammatory and hematopoietic recruitment in mice. Blood J Am Soc Hematol. 2013;122(24):3993–4001.
Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–58.
Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S, Bhatia M. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000;192(9):1365–72.
Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proceedings of the National Academy of Sciences 2007, 104(39):15436–15441.
Zhang P, Zhang C, Li J, Han J, Liu X, Yang H. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res Ther. 2019;10:1–13.
Beeraka NM, Basappa B, Nikolenko VN, Mahesh P. Role of Neurotransmitters in Steady State Hematopoiesis, Aging, and Leukemia. Stem Cell Reviews Rep 2024:1–26.
Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128–39.
Kang Q, Song W-X, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He B-C, Park JK. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18(4):545–58.
Deng Z-L, Sharff KA, Tang N, Song W-X, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13(1):2001–21.
Chen G, Deng C, Li Y-P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272.
Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang B, Zhang D, Rao P, Xiao J. PPARγ and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Therapy. 2016;11(3):216–25.
Park HW, Kim YC, Yu B, Moroishi T, Mo J-S, Plouffe SW, Meng Z, Lin KC, Yu F-X, Alexander CM. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162(4):780–94.
Byun M, Hwang J, Kim A, Kim K, Hwang E, Yaffe M, Hong J. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death Differ. 2014;21(6):854–63.
Bennett CN, Ouyang H, Ma YL, Zeng Q, Gerin I, Sousa KM, Lane TF, Krishnan V, Hankenson KD, MacDougald OA. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22(12):1924–32.
Stevens JR, Miranda-Carboni GA, Singer MA, Brugger SM, Lyons KM, Lane TF. Wnt10b deficiency results in age‐dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res. 2010;25(10):2138–47.
Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of β-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288(1):276–83.
Song B-q, Chi Y, Li X, Du W-j, Han Z-B, Tian J-j, Li J-j, Chen F, Wu H-h. Han L-x: Inhibition of Notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell Physiol Biochem. 2015;36(5):1991–2002.
Shimizu T, Tanaka T, Iso T, Matsui H, Ooyama Y, Kawai-Kowase K, Arai M, Kurabayashi M. Notch signaling pathway enhances bone morphogenetic protein 2 (BMP2) responsiveness of Msx2 gene to induce osteogenic differentiation and mineralization of vascular smooth muscle cells. J Biol Chem. 2011;286(21):19138–48.
Fontaine C, Cousin W, Plaisant M, Dani C, Peraldi P. Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells. 2008;26(4):1037–46.
Kim WK, Meliton V, Bourquard N, Hahn TJ, Parhami F. Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stress. J Cell Biochem. 2010;111(5):1199–209.
James AW, Pang S, Askarinam A, Corselli M, Zara JN, Goyal R, Chang L, Pan A, Shen J, Yuan W. Additive effects of sonic hedgehog and Nell-1 signaling in osteogenic versus adipogenic differentiation of human adipose-derived stromal cells. Stem Cells Dev. 2012;21(12):2170–8.
Li L, Dong Q, Wang Y, Feng Q, Zhou P, Ou X, Meng Q, He T, Luo J. Hedgehog signaling is involved in the BMP9-induced osteogenic differentiation of mesenchymal stem cells Retraction in/10.3892/ijmm. 2023.5233. International journal of molecular medicine 2015, 35(6):1641–1650.
Meunier P, Aaron J, Edouard C, VlGNON G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue: a quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Research®. 1971;80:147–54.
Justesen J, Stenderup K, Ebbesen E, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–71.
Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR‐γ2 transcription factor and TGF‐β/BMP signaling pathways. Aging Cell. 2004;3(6):379–89.
Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-γ expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284(40):27438–48.
Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone. 2012;50(2):437–43.
Schaum N, Lehallier B, Hahn O, Pálovics R, Hosseinzadeh S, Lee SE, Sit R, Lee DP, Losada PM, Zardeneta ME. Ageing hallmarks exhibit organ-specific temporal signatures. Nature. 2020;583(7817):596–602.
Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proceedings of the National Academy of Sciences 2010, 107(12):5465–5470.
Säwén P, Lang S, Mandal P, Rossi DJ, Soneji S, Bryder D. Mitotic history reveals distinct stem cell populations and their contributions to hematopoiesis. Cell Rep. 2016;14(12):2809–18.
Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, Passegué E. Autophagy maintains the metabolism and function of young and old stem cells. Nature. 2017;543(7644):205–10.
Florian MC, Dörr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, Filippi M-D, Hasenberg A, Gunzer M, Scharffetter-Kochanek K. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10(5):520–30.
Florian MC, Nattamai KJ, Dörr K, Marka G, Überle B, Vas V, Eckl C, Andrä I, Schiemann M, Oostendorp RA. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature. 2013;503(7476):392–6.
Dong S, Wang Q, Kao Y-R, Diaz A, Tasset I, Kaushik S, Thiruthuvanathan V, Zintiridou A, Nieves E, Dzieciatkowska M. Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Nature. 2021;591(7848):117–23.
Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, Haynes CM, Chen D. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347(6228):1374–7.
Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, Chen D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3(2):319–27.
Grigoryan A, Guidi N, Senger K, Liehr T, Soller K, Marka G, Vollmer A, Markaki Y, Leonhardt H, Buske C. LaminA/C regulates epigenetic and chromatin architecture changes upon aging of hematopoietic stem cells. Genome Biol. 2018;19(1):1–21.
Grigoryan A, Pospiech J, Krämer S, Lipka D, Liehr T, Geiger H, Kimura H, Mulaw MA, Florian MC. Attrition of x chromosome inactivation in aged hematopoietic stem cells. Stem Cell Rep. 2021;16(4):708–16.
Adelman ER, Huang H-T, Roisman A, Olsson A, Colaprico A, Qin T, Lindsley RC, Bejar R, Salomonis N, Grimes HL. Aging human hematopoietic stem cells manifest profound epigenetic reprogramming of enhancers that may predispose to leukemia. Cancer Discov. 2019;9(8):1080–101.
Mejia-Ramirez E, Florian MC. Understanding intrinsic hematopoietic stem cell aging. Haematologica. 2020;105(1):22.
Young K, Eudy E, Bell R, Loberg MA, Stearns T, Sharma D, Velten L, Haas S, Filippi M-D, Trowbridge JJ. Decline in IGF1 in the bone marrow microenvironment initiates hematopoietic stem cell aging. Cell Stem Cell. 2021;28(8):1473–82. e1477.
Cheng C-W, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TB. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810–23.
Matteini F, Mulaw MA, Florian MC. Aging of the hematopoietic stem cell niche: New tools to answer an old question. Front Immunol. 2021;12:738204.
Saçma M, Pospiech J, Bogeska R, de Back W, Mallm J-P, Sakk V, Soller K, Marka G, Vollmer A, Karns R. Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat Cell Biol. 2019;21(11):1309–20.
Shen B, Tasdogan A, Ubellacker JM, Zhang J, Nosyreva ED, Du L, Murphy MM, Hu S, Yi Y, Kara N. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature. 2021;591(7850):438–44.
Schürch CM, Riether C, Ochsenbein AF. Cytotoxic CD8 + T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell Stem Cell. 2014;14(4):460–72.
Yamashita M, Passegué E. TNF-α coordinates hematopoietic stem cell survival and myeloid regeneration. Cell Stem Cell. 2019;25(3):357–72. e357.
Valletta S, Thomas A, Meng Y, Ren X, Drissen R, Sengül H, Di Genua C, Nerlov C. Micro-environmental sensing by bone marrow stroma identifies IL-6 and TGFβ1 as regulators of hematopoietic ageing. Nat Commun. 2020;11(1):4075.
Comazzetto S, Shen B, Morrison SJ. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev Cell. 2021;56(13):1848–60.
Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45–56.
Geiger H, De Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13(5):376–89.
Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132(4):681–96.
de Haan G, Lazare SS. Aging of hematopoietic stem cells. Blood J Am Soc Hematol. 2018;131(5):479–87.
Watt SM, Hua P, Roberts I. Increasing complexity of molecular landscapes in human hematopoietic stem and progenitor cells during development and aging. Int J Mol Sci. 2022;23(7):3675.
Su T-Y, Hauenstein J, Somuncular E, Dumral Ö, Leonard E, Gustafsson C, Tzortzis E, Forlani A, Johansson A-S, Qian H. Aging is associated with functional and molecular changes in distinct hematopoietic stem cell subsets. Nat Commun. 2024;15(1):7966.
Sun D, Luo M, Jeong M, Rodriguez B, Xia Z, Hannah R, Wang H, Le T, Faull KF, Chen R. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014;14(5):673–88.
Kowalczyk MS, Tirosh I, Heckl D, Rao TN, Dixit A, Haas BJ, Schneider RK, Wagers AJ, Ebert BL, Regev A. Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells. Genome Res. 2015;25(12):1860–72.
Itokawa N, Oshima M, Koide S, Takayama N, Kuribayashi W, Nakajima-Takagi Y, Aoyama K, Yamazaki S, Yamaguchi K, Furukawa Y. Epigenetic traits inscribed in chromatin accessibility in aged hematopoietic stem cells. Nat Commun. 2022;13(1):2691.
Somuncular E, Hauenstein J, Khalkar P, Johansson A-S, Dumral Ö, Frengen NS, Gustafsson C, Mocci G, Su T-Y, Brouwer H. CD49b identifies functionally and epigenetically distinct subsets of lineage-biased hematopoietic stem cells. Stem Cell Rep. 2022;17(7):1546–60.
Arai F, Stumpf PS, Ikushima YM, Hosokawa K, Roch A, Lutolf MP, Suda T, MacArthur BD. Machine learning of hematopoietic stem cell divisions from paired daughter cell expression profiles reveals effects of aging on self-renewal. Cell Syst. 2020;11(6):640–52. e645.
Bowie MB, Kent DG, Dykstra B, McKnight KD, McCaffrey L, Hoodless PA, Eaves CJ. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proceedings of the National Academy of Sciences 2007, 104(14):5878–5882.
Kovtonyuk LV, Fritsch K, Feng X, Manz MG, Takizawa H. Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenvironment. Front Immunol. 2016;7:502.
Müller-Sieburg CE, Cho RH, Thoman M, Adkins B, Sieburg HB. Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood J Am Soc Hematol. 2002;100(4):1302–9.
Haas S, Trumpp A, Milsom MD. Causes and consequences of hematopoietic stem cell heterogeneity. Cell Stem Cell. 2018;22(5):627–38.
Kim KM, Mura-Meszaros A, Tollot M, Krishnan MS, Gründl M, Neubert L, Groth M, Rodriguez-Fraticelli A, Svendsen AF, Campaner S. Taz protects hematopoietic stem cells from an aging-dependent decrease in PU. 1 activity. Nat Commun. 2022;13(1):5187.
Lui JC, Chen W, Barnes KM, Baron J. Changes in gene expression associated with aging commonly originate during juvenile growth. Mech Ageing Dev. 2010;131(10):641–9.
Nerlov C, Graf T. PU. 1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12(15):2403–12.
Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL. PU. 1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J Exp Med. 2005;201(9):1487–502.
Metcalf D, Dakic A, Mifsud S, Di Rago L, Wu L, Nutt S. Inactivation of PU. 1 in adult mice leads to the development of myeloid leukemia. Proceedings of the National Academy of Sciences 2006, 103(5):1486–1491.
Voncken JW, van Schaick H, Kaartinen V, Deemer K, Coates T, Landing B, Pattengale P, Dorseuil O, Bokoch GM, Groffen J. Increased neutrophil respiratory burst in bcr-null mutants. Cell. 1995;80(5):719–28.
Khoury H, Barbara M, Iscove N, Minden M. Differential expression of the BCR gene in sequential stages of murine hematopoietic hierarchy. Leukemia. 2011;25(4):711–3.
Greuber EK, Smith-Pearson P, Wang J, Pendergast AM. Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer. 2013;13(8):559–71.
Cimmino L, Dawlaty MM, Ndiaye-Lobry D, Yap YS, Bakogianni S, Yu Y, Bhattacharyya S, Shaknovich R, Geng H, Lobry C. TET1 is a tumor suppressor of hematopoietic malignancy. Nat Immunol. 2015;16(6):653–62.
Joshi K, Zhang L, Breslin SJP, Kini AR, Zhang J. Role of TET dioxygenases in the regulation of both normal and pathological hematopoiesis. J Experimental Clin Cancer Res. 2022;41(1):294.
Blaney Davidson E, Scharstuhl A, Vitters E, Van Der Kraan P, Van Den Berg W. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res therapy. 2005;7:1–10.
Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153(4):828–39.
Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14(2):163–76.
Imamura T, Takase M, Nishihara A, Oeda E, Hanai J-i, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-β superfamily. Nature. 1997;389(6651):622–6.
Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood J Am Soc Hematol. 2010;115(16):3196–205.
Curran SP, Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3(4):e56.
Grewal SS. Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int J Biochem Cell Biol. 2009;41(5):1006–10.
Hamilton B, Dong Y, Shindo M, Liu W, Odell I, Ruvkun G, Lee SS. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19(13):1544–55.
Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6(1):111–9.
Greer EL, Maures TJ, Hauswirth AG, Green EM, Leeman DS, Maro GS, Han S, Banko MR, Gozani O, Brunet A. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466(7304):383–7.
Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, Benayoun BA, Shi Y, Brunet A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479(7373):365–71.
Liu L, Cheung TH, Charville GW, Hurgo BMC, Leavitt T, Shih J, Brunet A, Rando TA. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 2013;4(1):189–204.
Jeong M, Sun D, Luo M, Huang Y, Challen GA, Rodriguez B, Zhang X, Chavez L, Wang H, Hannah R. Large conserved domains of low DNA methylation maintained by Dnmt3a. Nat Genet. 2014;46(1):17–23.
Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, Bock C, Vasanthakumar A, Gu H, Xi Y. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2012;44(1):23–31.
Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363(25):2424–33.
Yan X-J, Xu J, Gu Z-H, Pan C-M, Lu G, Shen Y, Shi J-Y, Zhu Y-M, Tang L, Zhang X-W. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet. 2011;43(4):309–15.
Grover A, Sanjuan-Pla A, Thongjuea S, Carrelha J, Giustacchini A, Gambardella A, Macaulay I, Mancini E, Luis TC, Mead A. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat Commun. 2016;7(1):11075.
Martinez-Jimenez CP, Eling N, Chen H-C, Vallejos CA, Kolodziejczyk AA, Connor F, Stojic L, Rayner TF, Stubbington MJ, Teichmann SA. Aging increases cell-to-cell transcriptional variability upon immune stimulation. Science. 2017;355(6332):1433–6.
Mann M, Mehta A, de Boer C, Kowalczyk M, Lee K, Haldeman P, Rogel N, Knecht A, Farouq D, Regev A. Heterogeneous responses of hematopoietic stem cells to inflammatory stimuli are altered with age. Cell Rep. 2018;25:2992–3005. e5. In.
Ainciburu M, Ezponda T, Berastegui N, Alfonso-Pierola A, Vilas-Zornoza A, San Martin-Uriz P, Alignani D, Lamo-Espinosa J, San-Julian M, Jiménez-Solas T. Uncovering perturbations in human hematopoiesis associated with healthy aging and myeloid malignancies at single-cell resolution. Elife. 2023;12:e79363.
Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood J Am Soc Hematol. 2015;126(1):9–16.
Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, Thorgeirsson TE, Sigurdsson A, Gudjonsson SA, Gudmundsson J. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood J Am Soc Hematol. 2017;130(6):742–52.
Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472–8.
Mayle A, Yang L, Rodriguez B, Zhou T, Chang E, Curry CV, Challen GA, Li W, Wheeler D, Rebel VI. Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood. J Am Soc Hematol. 2015;125(4):629–38.
Busque L, Mio R, Mattioli J, Brais E, Blais N, Lalonde Y, Maragh M, Gilliland DG. Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. 1996.
Champion KM, Gilbert JG, Asimakopoulos FA, Hinshelwood S, Green AR. Clonal haemopoiesis in normal elderly women: implications for the myeloproliferative disorders and myelodysplastic syndromes. Br J Haematol. 1997;97(4):920–6.
van den Akker EB, Pitts SJ, Deelen J, Moed MH, Potluri S, van Rooij J, Suchiman HED, Lakenberg N, De Dijcker WJ, Uitterlinden AG. Uncompromised 10-year survival of oldest old carrying somatic mutations in DNMT3A and TET2. Blood. J Am Soc Hematol. 2016;127(11):1512–5.
Hoehn RS, Jernigan PL, Chang AL, Edwards MJ, Pritts TA. Molecular mechanisms of erythrocyte aging. Biol Chem. 2015;396(6–7):621–31.
Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu C-L, Sano S, Muralidharan S, Rius C. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842–7.
Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–21.
Rosendaal M, Adam J. Haemopoiesis by clonal succession? Blood Cells. 1984;10(2–3):473–85.
Roeder I, Kamminga LM, Braesel K, Dontje B, de Haan G, Loeffler M. Competitive clonal hematopoiesis in mouse chimeras explained by a stochastic model of stem cell organization. Blood. 2005;105(2):609–16.
Lu R, Neff NF, Quake SR, Weissman IL. Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat Biotechnol. 2011;29(10):928–33.
Gerrits A, Dykstra B, Kalmykowa OJ, Klauke K, Verovskaya E, Broekhuis MJ, de Haan G, Bystrykh LV. Cellular barcoding tool for clonal analysis in the hematopoietic system. Blood J Am Soc Hematol. 2010;115(13):2610–8.
Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho Y-J, Klein A, Hofmann O, Camargo FD. Clonal dynamics of native haematopoiesis. Nature. 2014;514(7522):322–7.
Vionnie W, Yusuf RZ, Oki T, Wu J, Saez B, Wang X, Cook C, Baryawno N, Ziller MJ, Lee E. Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell. 2016;167(5):1310–22. e1317.
Henninger J, Santoso B, Hans S, Durand E, Moore J, Mosimann C, Brand M, Traver D, Zon L. Clonal fate mapping quantifies the number of haematopoietic stem cells that arise during development. Nat Cell Biol. 2017;19(1):17–27.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–6.
Fadini GP, Avogaro A. It is all in the blood: the multifaceted contribution of circulating progenitor cells in diabetic complications. Experimental Diabetes Research 2012, 2012.
Hazra S, Jarajapu Y, Stepps V, Caballero S, Thinschmidt J, Sautina L, Bengtsson N, Licalzi S, Dominguez J, Kern T. Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model. Diabetologia. 2013;56:644–53.
Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol. 2010;105:703–12.
Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206(13):2897–906.
Ferraro F, Lymperi S, Méndez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3(104):ra104101–104101.
Albiero M, Poncina N, Tjwa M, Ciciliot S, Menegazzo L, Ceolotto G, Vigili de Kreutzenberg S, Moura R, Giorgio M, Pelicci P. Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes. 2014;63(4):1353–65.
Jha AK, Huang SC-C, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–30.
Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC-C, Griss T. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metabol. 2016;24(1):158–66.
Chen L-L, Morcelle C, Cheng Z-L, Chen X, Xu Y, Gao Y, Song J, Li Z, Smith MD, Shi M. Itaconate inhibits TET DNA dioxygenases to dampen inflammatory responses. Nat Cell Biol. 2022;24(3):353–63.
Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proceedings of the National Academy of Sciences 2013, 110(19):7820–7825.
Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62(17):1541–51.
van der Vorst EP, Weber C. Novel features of monocytes and macrophages in cardiovascular biology and disease. Arteriosclerosis, thrombosis, and vascular biology 2019, 39(2):e30–7.
Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo J-L, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125(2):364–74.
Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Reviews Cardiol. 2020;17(6):327–40.
Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circul Res. 2014;114(10):1611–22.
Rasheed A. Niche Regulation of Hematopoiesis: The Environment Is Micro, but the Influence Is Large. Arteriosclerosis, thrombosis, and vascular biology 2022, 42(6):691–699.
Campaner S, Doni M, Verrecchia A, Fagà G, Bianchi L, Amati B. Myc, Cdk2 and cellular senescence: Old players, new game. Cell Cycle. 2010;9(18):3679–85.
Nayak G, Odaka Y, Prasad V, Solano AF, Yeo E-J, Vemaraju S, Molkentin JD, Trumpp A, Williams B, Rao S. Developmental vascular regression is regulated by a Wnt/β-catenin, MYC and CDKN1A pathway that controls cell proliferation and cell death. Development. 2018;145(12):dev154898.
Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Domínguez Á, Pinho S, Akhmetzyanova I, Gao J, Witkowski M. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569(7755):222–8.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98.
Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, Schmid T, Brüne B, Wagner S, Serve H. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 2019;4(1):25–33.
Svensson EC, Madar A, Campbell CD, He Y, Sultan M, Healey ML, D’Aco K, Fernandez A, Wache-Mainier C, Ridker PM. TET2-driven clonal hematopoiesis predicts enhanced response to canakinumab in the CANTOS trial: an exploratory analysis. Circulation. 2018;138(Suppl1):A15111–15111.
Hoffmann J, Luxán G, Abplanalp WT, Glaser S-F, Rasper T, Fischer A, Muhly-Reinholz M, Potente M, Assmus B, John D. Post-myocardial infarction heart failure dysregulates the bone vascular niche. Nat Commun. 2021;12(1):3964.
Caiado F, Pietras EM, Manz MG. Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J Exp Med. 2021;218(7):e20201541.
Bick AG, Weinstock JS, Nandakumar SK, Fulco CP, Bao EL, Zekavat SM, Szeto MD, Liao X, Leventhal MJ, Nasser J. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature. 2020;586(7831):763–8.
Cook EK, Izukawa T, Young S, Rosen G, Jamali M, Zhang L, Johnson D, Bain E, Hilland J, Ferrone CK. Comorbid and inflammatory characteristics of genetic subtypes of clonal hematopoiesis. Blood Adv. 2019;3(16):2482–6.
Zhou L, McMahon C, Bhagat T, Alencar C, Yu Y, Fazzari M, Sohal D, Heuck C, Gundabolu K, Ng C. Reduced SMAD7 leads to overactivation of TGF-β signaling in MDS that can be reversed by a specific inhibitor of TGF-β receptor I kinase. Cancer Res. 2011;71(3):955–63.
Fenaux P, Kiladjian JJ, Platzbecker U. Luspatercept for the treatment of anemia in myelodysplastic syndromes and primary myelofibrosis. Blood. J Am Soc Hematol. 2019;133(8):790–4.
Arranz L, Sánchez-Aguilera A, Martín-Pérez D, Isern J, Langa X, Tzankov A, Lundberg P, Muntión S, Tzeng Y-S, Lai D-M. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78–81.
Kovtun I, von Bonin M, Ibneeva L, Frimmel J, Middeke JM, Kunadt D, Heberling L, Wobus M, Bornhäuser M, Grinenko T. Profound sympathetic neuropathy in the bone marrow of patients with acute myeloid leukemia. Leukemia. 2024;38(2):393–7.
Mistry JJ, Young KA, Trowbridge JJ. Bone Marrow Stromal Cell Senescence Induced By Dnmt3a-Mutant Hematopoietic Stem and Progenitor Cells Accelerates Clonal Hematopoiesis and Progression to Leukemia. Blood. 2022;140(Supplement 1):1265–1265.
Nguyen YT, Fujisawa M, Nguyen TB, Suehara Y, Sakamoto T, Matsuoka R, Abe Y, Fukumoto K, Hattori K, Noguchi M. Tet2 deficiency in immune cells exacerbates tumor progression by increasing angiogenesis in a lung cancer model. Cancer Sci. 2021;112(12):4931–43.
Abplanalp WT, Cremer S, John D, Hoffmann J, Schuhmacher B, Merten M, Rieger MA, Vasa-Nicotera M, Zeiher AM, Dimmeler S. Clonal hematopoiesis–driver DNMT3A mutations alter immune cells in heart failure. Circul Res. 2021;128(2):216–28.
Zioni N, Bercovich AA, Chapal-Ilani N, Bacharach T, Rappoport N, Solomon A, Avraham R, Kopitman E, Porat Z, Sacma M. Inflammatory signals from fatty bone marrow support DNMT3A driven clonal hematopoiesis. Nat Commun. 2023;14(1):2070.
Abegunde SO, Buckstein R, Wells RA, Rauh MJ. An inflammatory environment containing TNFα favors Tet2-mutant clonal hematopoiesis. Exp Hematol. 2018;59:60–5.
Liao M, Chen R, Yang Y, He H, Xu L, Jiang Y, Guo Z, He W, Jiang H, Wang J. Aging-elevated inflammation promotes DNMT3A R878H-driven clonal hematopoiesis. Acta Pharm Sinica B. 2022;12(2):678–91.
Boy M, Bisio V, Zhao L-P, Guidez F, Schell B, Lereclus E, Henry G, Villemonteix J, Rodrigues-Lima F, Gagne K. Myelodysplastic Syndrome associated TET2 mutations affect NK cell function and genome methylation. Nat Commun. 2023;14(1):588.
Winter S, Götze KS, Hecker JS, Metzeler KH, Guezguez B, Woods K, Medyouf H, Schäffer A, Schmitz M, Wehner R. Clonal hematopoiesis and its impact on the aging osteo-hematopoietic niche. Leukemia. 2024;38(5):936–46.
Acknowledgements
Our sincere thanks to department staff of Human Anatomy and Histology, Sechenov University, Moscow, Russia.
Funding
This study was supported by Herman Wells Pediatric Research Center, Indianapolis, USA.
Author information
Authors and Affiliations
Contributions
Yang Xinyi (YX), Reshetov Igor Vladimirovich (RIV), Narasimha M Beeraka (NMB), Allaka Satyavathi (AS), Dinisha Kamble (DK), Vladimir N Nikolenko (VNN), Allaka Naga Lakshmi (ANL), Basappa Basappa (BB), Padmanabha Reddy Y (PRY), Ruitai Fan (RF), Junqi Liu (JL) designed the concept, and YX (Primary author) NMB (Main corresponding author) and RF wrote the manuscript; NMB, YX proofread, edited, and analyzed the content of the article. All authors reviewed the manuscript and approved it before submission.
Corresponding authors
Ethics declarations
Ethics approval
NA.
Consent for publication
NA.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xinyi, Y., Vladimirovich, R.I., Beeraka, N.M. et al. Emerging insights into epigenetics and hematopoietic stem cell trafficking in age-related hematological malignancies. Stem Cell Res Ther 15, 401 (2024). https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s13287-024-04008-4
Received:
Accepted:
Published:
DOI: https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s13287-024-04008-4